The Definitive Guide to Medical Parts Feeding Systems: Cost, Materials & Top Vendors
Introduction: Navigating the Global Market for medical parts feeding systems
In the ever-evolving landscape of the medical industry, sourcing reliable medical parts feeding systems presents a significant challenge for international B2B buyers. As manufacturers grapple with stringent regulatory requirements and the demand for increased efficiency, the need for precision, adaptability, and hygiene in feeding systems has never been more critical. This comprehensive guide delves into the myriad types of medical parts feeding systems available, their applications in various manufacturing processes, and the importance of selecting the right supplier. By exploring factors such as system design, material compatibility, and cost considerations, this guide empowers buyers from regions like Africa, South America, the Middle East, and Europe—including key markets like Brazil and Nigeria—to make informed purchasing decisions that enhance operational efficiency and product quality.
Throughout this guide, we will provide insights into the latest technological innovations in medical feeding systems, highlight the benefits of flexibility and modularity, and discuss best practices for supplier vetting. With the right information at their fingertips, B2B buyers can navigate the complexities of the global market and position their organizations for success in an increasingly competitive environment. Ultimately, this guide serves as a vital resource for those seeking to optimize their production lines and ensure the seamless integration of medical parts feeding systems into their manufacturing processes.
Understanding medical parts feeding systems Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Vibratory Feeders | Utilizes vibration to move parts; suitable for bulk feeding | Pharmaceutical packaging, assembly lines | Pros: High speed, low maintenance; Cons: Can be noisy, may require calibration. |
| Centrifugal Feeders | Uses centrifugal force for part orientation and movement | Handling bottles, vials, and caps | Pros: High efficiency, precise orientation; Cons: Limited to specific part shapes. |
| Flexible Feeders | Adapts to various part shapes and sizes; modular design | Medical device assembly, quality control | Pros: Versatile, reduces downtime; Cons: Higher initial investment. |
| Bowl Feeders | Circular bowl design for orienting and feeding parts | Small component assembly, packaging | Pros: Effective for small parts, customizable; Cons: Limited to specific applications. |
| Conveyor Systems | Continuous movement of parts along a conveyor belt | Transporting items through production lines | Pros: Streamlined processes, scalable; Cons: Space-consuming, may require integration with other systems. |
What Are Vibratory Feeders and Their Suitability for Medical Applications?
Vibratory feeders are designed to utilize vibrations to move and orient parts, making them ideal for bulk feeding applications. Their ability to handle a wide range of materials and parts, including delicate pills and small vials, makes them a popular choice in pharmaceutical packaging and assembly lines. When considering vibratory feeders, buyers should evaluate the noise levels and maintenance requirements, as these factors can affect workplace efficiency and costs.
How Do Centrifugal Feeders Work and Where Are They Most Effective?
Centrifugal feeders leverage centrifugal force to effectively orient and move parts, making them particularly suitable for handling items like bottles, vials, and caps. Their high efficiency and precise orientation capabilities are essential in fast-paced environments where accuracy is critical. For B2B buyers, understanding the limitations in terms of part shape compatibility is crucial, as these systems may not be suitable for all component types.
Why Choose Flexible Feeders for Diverse Medical Manufacturing Needs?
Flexible feeders, such as the innovative FlexiBowl, are designed to adapt to various part shapes and sizes, making them essential in dynamic manufacturing environments. These systems are especially beneficial for medical device assembly and quality control processes, where rapid changes in part types may occur. While they offer significant advantages in versatility and reduced downtime, potential buyers should be prepared for a higher initial investment compared to traditional feeders.
Illustrative image related to medical parts feeding systems
What Are the Advantages of Bowl Feeders in Medical Parts Feeding?
Bowl feeders feature a circular design that allows for efficient orienting and feeding of small parts, making them suitable for applications such as component assembly and packaging in the medical sector. They can be customized to handle specific parts, enhancing their effectiveness. However, buyers should consider their limited application range and ensure that the feeder’s capabilities align with their operational needs.
How Do Conveyor Systems Enhance Production Efficiency in Medical Manufacturing?
Conveyor systems provide a continuous movement solution for transporting parts along production lines, facilitating streamlined processes in medical manufacturing. Their scalability allows for integration into various production setups, improving overall efficiency. However, buyers must be aware of the space requirements and potential need for integration with other systems, as these factors can impact the overall cost and layout of the production facility.
Key Industrial Applications of medical parts feeding systems
| Industry/Sector | Specific Application of medical parts feeding systems | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Pharmaceutical | Automated feeding of pills and capsules for packaging | Increases production speed while ensuring accuracy | Compliance with regulatory standards, cleanroom compatibility, and system flexibility. |
| Medical Device Manufacturing | Assembly line support for various medical device components | Enhances workflow efficiency and reduces assembly errors | Adaptability to handle multiple part shapes and sizes, quick changeover capabilities. |
| Disposable Medical Products | Feeding and packaging of syringes, gloves, and bandages | Streamlines production processes and reduces downtime | Material compatibility, hygiene standards, and system reliability. |
| Diagnostic Equipment | Orientation and feeding of small electronic components | Improves quality control and precision in assembly | Precision handling, integration with existing systems, and maintenance support. |
| Laboratory Equipment | Feeding of vials and sample containers for testing | Ensures consistent supply and reduces manual handling | Cleanroom design, automation capabilities, and ease of integration. |
How Are Medical Parts Feeding Systems Used in the Pharmaceutical Industry?
In the pharmaceutical sector, medical parts feeding systems are crucial for automating the feeding and packaging of pills and capsules. These systems help maintain high production speeds while ensuring the accuracy and integrity of the products. For international buyers, especially in regions like Africa and South America, sourcing equipment that complies with stringent regulatory standards and fits cleanroom requirements is essential. Buyers should consider the flexibility of these systems to adapt to various product types and sizes.
What Role Do Feeding Systems Play in Medical Device Manufacturing?
Medical device manufacturers utilize feeding systems to streamline the assembly of diverse components, such as surgical instruments and diagnostic devices. By integrating these systems into production lines, businesses can enhance workflow efficiency and minimize assembly errors. For B2B buyers in Europe and the Middle East, it’s vital to select equipment that can quickly adapt to different part shapes and sizes, ensuring rapid changeovers without compromising quality.
How Do Feeding Systems Optimize Production for Disposable Medical Products?
In the production of disposable medical products like syringes and gloves, feeding systems facilitate efficient handling and packaging. These systems minimize manual intervention, thereby reducing the risk of contamination and increasing throughput. Buyers from regions like Nigeria and Brazil should prioritize sourcing systems that meet hygiene standards and are made from materials suitable for medical applications to ensure safety and compliance.
What Benefits Do Feeding Systems Provide in Diagnostic Equipment Production?
Feeding systems are employed in the assembly of diagnostic equipment, particularly for orienting and feeding small electronic components. These systems enhance quality control by ensuring precise positioning and handling of delicate parts. International buyers must focus on sourcing systems that offer precision handling and can integrate seamlessly with existing assembly lines, thereby improving overall productivity.
How Are Medical Parts Feeding Systems Used in Laboratory Equipment Production?
In laboratory settings, feeding systems play a pivotal role in supplying vials and sample containers for testing processes. These systems ensure a consistent supply of components while reducing the need for manual handling, which can lead to contamination. For B2B buyers, especially in emerging markets, it is crucial to consider systems designed for cleanroom environments and those that offer reliable automation capabilities to enhance operational efficiency.
3 Common User Pain Points for ‘medical parts feeding systems’ & Their Solutions
Scenario 1: Struggling with Component Orientation and Handling
The Problem: In the medical manufacturing environment, ensuring precise orientation of delicate components is critical. B2B buyers often encounter issues with standard feeders that cannot adequately handle the diverse shapes and sizes of medical parts, such as syringes or vials. This misalignment can lead to increased downtime, production errors, and costly waste. The challenge is particularly pronounced in fast-paced settings where quick changeovers are necessary due to varying product lines and stringent regulatory standards.
The Solution: To overcome these challenges, buyers should consider investing in advanced flexible feeding systems like the FlexiBowl®. This system is designed for versatility, allowing it to adapt to various component shapes and sizes without extensive downtime for changeovers. When sourcing, look for systems that provide features such as pneumatic operation suitable for cleanroom environments and precise part orientation capabilities. Ensuring the chosen system has a proven track record in similar applications can greatly enhance workflow efficiency. Additionally, collaborating with suppliers who offer customization options can help in tailoring the system to meet specific operational needs.
Scenario 2: Compliance with Strict Regulatory Standards
The Problem: Medical parts feeding systems must adhere to rigorous regulatory standards, which can vary significantly across different regions, such as Europe and Africa. B2B buyers often struggle with ensuring that their feeding systems not only meet local regulations but also maintain the highest hygiene and quality standards. Failing to comply can lead to severe penalties, product recalls, and damage to a company’s reputation.
The Solution: Buyers should prioritize sourcing equipment that is certified for compliance with international quality standards, such as ISO 14644-1 for cleanroom environments. When evaluating suppliers, ask for detailed documentation of compliance and any relevant certifications. It is also beneficial to work with suppliers who offer comprehensive service and support, including training on regulatory requirements and best practices for maintaining compliance. Investing in systems with built-in sanitation features, such as stainless steel construction and easy-to-clean designs, can simplify compliance efforts and ensure that manufacturing processes meet necessary hygiene standards.
Scenario 3: Integrating New Technologies into Existing Systems
The Problem: The rapid evolution of technology in the medical sector often leaves companies struggling to integrate new feeding systems into their existing production lines. B2B buyers may find themselves facing compatibility issues with legacy systems, leading to operational disruptions and increased costs. Additionally, the challenge of training staff on new technologies can further complicate the transition.
The Solution: To facilitate seamless integration, buyers should look for modular and flexible feeding systems that are designed for easy incorporation into existing workflows. When selecting a system, opt for solutions that offer compatibility with Industry 4.0 technologies, such as IoT capabilities, which can enhance monitoring and data collection. It is also crucial to engage suppliers who provide extensive training and support during the implementation phase. This can include on-site training sessions for staff and ongoing technical support to address any challenges that arise post-installation. By taking these proactive steps, companies can ensure a smoother transition and maximize the return on their investment in new feeding technologies.
Strategic Material Selection Guide for medical parts feeding systems
What Are the Key Materials Used in Medical Parts Feeding Systems?
In the realm of medical parts feeding systems, the choice of materials is crucial due to the stringent requirements for hygiene, durability, and performance. Below, we analyze four common materials used in these systems, highlighting their properties, advantages, disadvantages, and considerations for international B2B buyers.

Illustrative image related to medical parts feeding systems
How Does Stainless Steel Perform in Medical Parts Feeding Systems?
Key Properties: Stainless steel is renowned for its excellent corrosion resistance, high strength, and ability to withstand high temperatures and pressures. It typically meets standards such as ASTM A240 and ISO 5832, making it suitable for medical applications.
Pros & Cons: The durability of stainless steel ensures a long service life, reducing the need for frequent replacements. However, it can be more expensive than other materials, and its manufacturing complexity may increase costs. Stainless steel is ideal for environments requiring high cleanliness standards, but it may not be suitable for highly corrosive substances.
Impact on Application: Stainless steel is compatible with a wide range of media, including various pharmaceuticals and biological substances. Its non-reactive nature ensures that it does not contaminate sensitive materials, making it a preferred choice for feeding systems in cleanrooms.
Considerations for International Buyers: Buyers from regions like Africa and South America should ensure compliance with local regulations regarding material safety and hygiene. Understanding the specific standards such as ASTM or DIN is essential for ensuring product quality and acceptance in the market.
What Role Does FDA-Approved Plastics Play in Medical Parts Feeding Systems?
Key Properties: FDA-approved plastics, such as polypropylene (PP) and polycarbonate (PC), offer good chemical resistance and are lightweight. They are often rated for use in food and medical applications, ensuring safety and compliance.

Illustrative image related to medical parts feeding systems
Pros & Cons: The primary advantage of these plastics is their cost-effectiveness and ease of manufacturing. They can be molded into complex shapes, which is beneficial for custom feeding solutions. However, they may have lower temperature resistance compared to metals, limiting their use in high-heat applications.
Impact on Application: These materials are suitable for handling non-corrosive liquids and solids, making them ideal for feeding systems that work with medications and medical devices. Their lightweight nature also facilitates faster production speeds.
Considerations for International Buyers: It is crucial for buyers to verify that the plastics meet local health and safety regulations. Different countries may have varying standards for FDA compliance, which can affect product acceptance.
How Do Elastomers Enhance the Functionality of Medical Parts Feeding Systems?
Key Properties: Elastomers, such as silicone and rubber, provide excellent flexibility and resilience. They can operate effectively in a range of temperatures and are often resistant to various chemicals.
Pros & Cons: The flexibility of elastomers allows for effective sealing and cushioning, making them suitable for applications requiring vibration dampening. However, they may wear out faster than metals, leading to higher replacement costs over time.

Illustrative image related to medical parts feeding systems
Impact on Application: Elastomers are often used in gaskets and seals within feeding systems, ensuring that no contaminants enter the product stream. Their compatibility with a variety of media makes them versatile for different medical applications.
Considerations for International Buyers: Buyers must consider the specific chemical compatibility of elastomers with the substances they handle. Additionally, ensuring that these materials meet international standards for medical use is critical for compliance.
What Advantages Do Composite Materials Offer in Medical Parts Feeding Systems?
Key Properties: Composite materials combine the benefits of different materials, such as plastics and metals, to achieve specific performance characteristics. They can be engineered for high strength-to-weight ratios and tailored chemical resistance.
Pros & Cons: The main advantage of composites is their ability to be customized for specific applications, providing a balance of durability and lightweight properties. However, they can be more expensive to produce and may require specialized manufacturing processes.
Impact on Application: Composites are particularly useful in applications where weight is a concern, such as portable medical devices. Their tailored properties can enhance performance in specific environments, such as humid or chemically aggressive settings.
Considerations for International Buyers: As composite materials may not be universally recognized, buyers should ensure that they meet local standards and certifications. Understanding the specific requirements for composites in different regions is essential for successful procurement.
Summary Table of Material Selection for Medical Parts Feeding Systems
| Material | Typical Use Case for medical parts feeding systems | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Stainless Steel | Feeding systems in cleanroom environments | High durability and corrosion resistance | Higher cost and manufacturing complexity | High |
| FDA-Approved Plastics | Handling non-corrosive liquids and solids | Cost-effective and easy to mold | Lower temperature resistance | Medium |
| Elastomers | Seals and gaskets in feeding systems | Excellent flexibility and resilience | Potential for faster wear | Medium |
| Composite Materials | Lightweight applications in portable devices | Customizable performance characteristics | Higher production costs | High |
This strategic material selection guide provides valuable insights for international B2B buyers, enabling informed decisions that align with both operational needs and regulatory requirements.
In-depth Look: Manufacturing Processes and Quality Assurance for medical parts feeding systems
What Are the Main Stages of Manufacturing Medical Parts Feeding Systems?
Manufacturing medical parts feeding systems involves a series of meticulously controlled stages to ensure both efficiency and compliance with rigorous industry standards. The primary stages include material preparation, forming, assembly, and finishing.
How is Material Prepared for Medical Parts Feeding Systems?
Material preparation is crucial in ensuring that the components meet health and safety requirements. This stage typically involves sourcing high-grade materials such as stainless steel, which is favored for its durability and resistance to corrosion. In addition, the materials must undergo thorough inspection upon receipt, often involving visual checks and dimensional analysis to confirm compliance with specifications.
Illustrative image related to medical parts feeding systems
What Forming Techniques Are Commonly Used in the Production of Medical Parts Feeding Systems?
The forming stage employs various techniques to create components that are both functional and compliant with medical regulations. Common methods include:
- Machining: This includes processes like milling, turning, and grinding to achieve precise dimensions.
- Injection Molding: Often used for plastic components, this technique allows for high-volume production while maintaining consistency in quality.
- Stamping: This is frequently applied for metal parts, enabling the production of complex shapes with minimal waste.
Each technique must be chosen based on the specific requirements of the medical parts being produced, ensuring that the resulting components meet stringent quality and safety standards.
What Does the Assembly Process Entail for Medical Parts Feeding Systems?
The assembly process is where individual components come together to form the final feeding system. This stage often requires specialized equipment and skilled labor to ensure that each part is correctly positioned and secured. Key considerations during assembly include:
- Cleanroom Environments: Many medical parts feeding systems require assembly in controlled environments to prevent contamination. ISO Class 5 cleanrooms are commonly used, meeting standards defined by ISO 14644-1.
- Precision Alignment: Advanced tools such as automated vision systems may be used to ensure that components are correctly oriented and assembled, minimizing the risk of errors.
How Is Finishing Achieved in the Production of Medical Parts Feeding Systems?
Finishing processes are essential for enhancing the functionality and appearance of medical parts feeding systems. Common finishing techniques include:
- Surface Treatment: Processes like passivation or anodizing improve corrosion resistance and hygiene.
- Cleaning: Components undergo rigorous cleaning processes to eliminate any contaminants before they are packaged for shipment.
- Final Inspection: A thorough final inspection is conducted to ensure that all components meet the required specifications and quality standards.
What Quality Assurance Measures Are Essential for Medical Parts Feeding Systems?
Quality assurance (QA) is integral to the manufacturing of medical parts feeding systems. It ensures that products not only meet customer expectations but also comply with regulatory requirements.
Illustrative image related to medical parts feeding systems
Which International Standards Should B2B Buyers Be Aware of?
B2B buyers should familiarize themselves with several international and industry-specific standards that govern the manufacturing of medical devices:
- ISO 9001: This standard focuses on quality management systems, emphasizing continuous improvement and customer satisfaction.
- ISO 13485: Specifically tailored for the medical device industry, this standard outlines requirements for a quality management system that ensures consistent product quality.
- CE Marking: In Europe, the CE mark indicates conformity with health, safety, and environmental protection standards.
- API Standards: For certain medical applications, adherence to the standards set forth by the American Petroleum Institute (API) may be necessary.
Understanding these standards can help buyers assess the credibility and reliability of potential suppliers.
What Are the Key Quality Control Checkpoints Throughout the Manufacturing Process?
Quality control (QC) checkpoints are strategically integrated throughout the manufacturing process to catch defects early. Common checkpoints include:
- Incoming Quality Control (IQC): This involves inspecting raw materials upon receipt to ensure they meet specified requirements.
- In-Process Quality Control (IPQC): Conducted at various stages of production, IPQC helps identify defects during manufacturing, allowing for immediate corrective actions.
- Final Quality Control (FQC): A comprehensive review of finished products ensures that they meet all quality standards before being shipped to clients.
Implementing these checkpoints not only enhances product quality but also reduces the risk of costly recalls.
How Can B2B Buyers Verify Supplier Quality Control?
Verifying the quality control processes of suppliers is essential for B2B buyers looking to ensure that they are sourcing reliable medical parts feeding systems. Here are several strategies:

Illustrative image related to medical parts feeding systems
What Should Buyers Look for During Supplier Audits?
Conducting supplier audits provides an in-depth understanding of a supplier’s manufacturing processes and quality assurance measures. During audits, buyers should assess:
- Documentation: Review the supplier’s quality management system documentation, including procedures, policies, and training records.
- Process Observations: Observe the manufacturing processes to evaluate compliance with established standards and practices.
- Employee Interviews: Engaging with employees can provide insights into the organization’s commitment to quality.
What Types of Reports Should Buyers Request?
Buyers should request various quality reports to gain a comprehensive view of a supplier’s QC practices. Key reports include:
- Quality Metrics: Data on defect rates, corrective actions taken, and overall performance against established KPIs.
- Compliance Certificates: Documentation proving adherence to relevant standards (e.g., ISO certifications).
- Inspection Reports: Detailed reports from third-party inspections, which can provide an unbiased assessment of quality practices.
What Are the Nuances of Quality Control for International B2B Buyers?
For international buyers, particularly those from regions like Africa, South America, the Middle East, and Europe, understanding the nuances of quality control is vital. These may include:
- Regulatory Differences: Different regions have varying regulatory requirements. Buyers must ensure that their suppliers comply with local regulations in their respective markets.
- Cultural Considerations: Cultural differences may impact communication and quality practices. Establishing clear communication channels is crucial for successful collaboration.
- Logistics and Supply Chain Management: Understanding the logistics involved in shipping and customs can impact the timely delivery of quality products.
By taking these factors into account, international B2B buyers can make informed decisions and build strong, reliable partnerships with suppliers of medical parts feeding systems.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘medical parts feeding systems’
Introduction
In the rapidly evolving medical sector, sourcing the right medical parts feeding system is crucial for maintaining efficiency and compliance. This guide provides a structured checklist to help B2B buyers navigate the complexities of procuring feeding systems tailored for medical applications. By following these steps, you can ensure that your organization selects a reliable and effective solution that meets your specific needs.
Step 1: Define Your Technical Specifications
Start by clearly outlining the technical requirements for your feeding system. Consider factors such as the types of components to be handled (e.g., vials, syringes, or capsules) and the desired throughput rates.
- Compatibility: Ensure that the system can integrate with your existing production line.
- Cleanroom Standards: Specify cleanliness levels necessary for your production environment, such as ISO Class 5 or higher.
Step 2: Assess Regulatory Compliance
Verify that potential suppliers adhere to relevant industry regulations and standards. Medical manufacturing is subject to strict guidelines, so ensuring compliance is essential for quality assurance.
- Certification Verification: Request documentation for certifications like ISO 13485 or FDA compliance.
- Track Record: Inquire about the supplier’s history in passing regulatory audits and their commitment to quality control.
Step 3: Evaluate Potential Suppliers
Before committing, thoroughly vet suppliers to ensure they can meet your requirements. A reliable supplier should demonstrate experience and expertise in the medical sector.
- Request Documentation: Ask for company profiles, case studies, and references from similar industries or regions.
- Site Visits: If possible, conduct site visits to observe their operations and quality control processes.
Step 4: Analyze Equipment Flexibility and Adaptability
Given the dynamic nature of the medical industry, choose a feeding system that offers flexibility and can adapt to changing production needs.
- Modularity: Look for systems that allow easy configuration for different part types and sizes.
- Technology Integration: Ensure the system can incorporate Industry 4.0 technologies for enhanced efficiency and data management.
Step 5: Investigate After-Sales Support and Maintenance Services
A strong support system is vital for the longevity and efficiency of your feeding system. Investigate the level of after-sales support offered by suppliers.
- Service Agreements: Understand the terms of service agreements, including response times for maintenance and repairs.
- Training Programs: Check if the supplier provides training for your staff to ensure effective operation of the feeding systems.
Step 6: Request Demonstrations and Trials
Whenever possible, request demonstrations or trials of the feeding systems you are considering. This hands-on experience can provide valuable insights into the system’s performance and suitability for your application.

Illustrative image related to medical parts feeding systems
- Performance Metrics: Evaluate the system’s efficiency, speed, and accuracy during the demonstration.
- Feedback from Operators: Gather input from your team about the ease of use and any potential challenges they foresee.
Step 7: Compare Costs and Total Cost of Ownership
Finally, analyze the cost of different systems, but also consider the total cost of ownership, which includes maintenance, training, and potential downtime.
- Initial Costs vs. Long-Term Savings: A more expensive system may offer greater reliability and efficiency, leading to lower operational costs over time.
- Financing Options: Explore financing options or leasing agreements that may help manage cash flow while investing in quality equipment.
By following this checklist, you can confidently navigate the sourcing process for medical parts feeding systems, ensuring that you select a solution that aligns with your operational needs and regulatory requirements.
Comprehensive Cost and Pricing Analysis for medical parts feeding systems Sourcing
What Are the Key Cost Components of Medical Parts Feeding Systems?
When sourcing medical parts feeding systems, understanding the cost structure is crucial for B2B buyers. The primary cost components include:

Illustrative image related to medical parts feeding systems
-
Materials: The choice of materials significantly impacts costs. Stainless steel is commonly used for its durability and compliance with hygiene standards, but it may come at a premium. The specific materials used will depend on the required certifications and the environment in which the systems will operate.
-
Labor: Labor costs encompass both direct labor for assembly and indirect labor for design, programming, and maintenance. Skilled technicians are essential for high-precision assembly, particularly in the medical sector where quality is paramount.
-
Manufacturing Overhead: This includes costs associated with running the production facility, such as utilities, equipment depreciation, and facility maintenance. High-quality manufacturing environments often require advanced machinery and cleanroom standards, which can elevate overhead costs.
-
Tooling: Custom tooling may be required for specialized components or systems. The investment in tooling is often amortized over larger production runs, so buyers should consider the implications of their order volume on this cost.
-
Quality Control (QC): Rigorous QC processes are essential in the medical industry. Costs associated with testing, inspections, and certifications ensure compliance with regulatory standards, which can be significant, particularly for high-risk medical devices.
-
Logistics: Shipping costs can vary widely based on distance, shipping methods, and customs duties. International buyers must account for potential delays and tariffs that could affect overall costs.
-
Margin: Suppliers typically include a profit margin in their pricing. Understanding the market rate for similar systems can help buyers negotiate effectively.
How Do Price Influencers Affect Medical Parts Feeding Systems?
Several factors can influence the pricing of medical parts feeding systems, including:
-
Volume/MOQ: Suppliers often provide better pricing for larger orders due to economies of scale. Minimum order quantities (MOQs) can also affect pricing; understanding the supplier’s MOQ is essential for cost management.
-
Specifications and Customization: Custom solutions tailored to specific needs generally come at a higher price point. If a buyer requires specialized features or materials, this can significantly influence the overall cost.
-
Materials and Quality Certifications: Higher-quality materials and certifications (like ISO compliance) can increase costs but are often necessary for regulatory compliance in the medical field. Buyers should weigh the importance of these factors against their budget.
-
Supplier Factors: The reputation and reliability of the supplier can impact pricing. Established suppliers with a track record of quality may charge more, but they also bring reliability and expertise.
-
Incoterms: The chosen Incoterms (International Commercial Terms) can affect pricing and risk. Buyers should clarify which party is responsible for shipping, insurance, and customs to avoid unexpected costs.
What Tips Can Help Buyers Negotiate Effectively?
For international B2B buyers, particularly from Africa, South America, the Middle East, and Europe, negotiating effectively can lead to cost savings. Here are some strategies:
-
Research and Benchmarking: Compare prices from multiple suppliers to establish a baseline. Understanding the typical price range for similar systems can empower buyers during negotiations.
-
Total Cost of Ownership (TCO): Consider not just the initial purchase price but the entire lifecycle cost of the system, including maintenance, operational efficiency, and downtime. A higher upfront cost may be justified if it results in lower operational costs over time.
-
Flexibility in Specifications: If possible, remain open to alternative specifications that may reduce costs. This could involve less stringent material requirements or standardized components.
-
Long-Term Relationships: Establishing a long-term partnership with suppliers can lead to better pricing and terms. Suppliers are often willing to negotiate for repeat business.
-
Understanding Pricing Nuances: International buyers should be aware of currency fluctuations, regional pricing strategies, and local market conditions that can affect overall costs.
Disclaimer
Prices for medical parts feeding systems can vary widely based on the factors discussed above. The information provided is for indicative purposes only and should not be construed as a fixed price. Buyers are encouraged to conduct thorough market research and engage in direct discussions with suppliers for accurate pricing.
Alternatives Analysis: Comparing medical parts feeding systems With Other Solutions
Exploring Alternative Solutions to Medical Parts Feeding Systems
When evaluating the best approach for handling medical components, it is essential to consider various alternatives to traditional medical parts feeding systems. These alternatives can provide different benefits depending on the specific needs of the manufacturing process, including flexibility, efficiency, and cost-effectiveness. Below is a comparative analysis of medical parts feeding systems against two viable alternatives: automated robotic pick-and-place systems and manual feeding techniques.
| Comparison Aspect | Medical Parts Feeding Systems | Automated Robotic Pick-and-Place Systems | Manual Feeding Techniques |
|---|---|---|---|
| Performance | High precision and speed for handling delicate parts | Very high speed with flexibility in part handling | Lower speed and higher variability in precision |
| Cost | Moderate to high initial investment | High initial cost but can lead to long-term savings | Low initial cost but high labor costs |
| Ease of Implementation | Requires integration with existing systems | Complex integration and programming needed | Simple setup with minimal technology |
| Maintenance | Regular maintenance needed for optimal performance | Requires skilled technicians for repairs | Minimal maintenance but subject to human error |
| Best Use Case | Ideal for high-volume production of standardized components | Best for highly flexible and complex production lines | Suitable for low-volume or highly customized operations |
What Are the Pros and Cons of Automated Robotic Pick-and-Place Systems?
Automated robotic pick-and-place systems are designed for high-speed operations and can handle a variety of components, making them a strong alternative to traditional feeding systems. Their primary advantage lies in their flexibility; they can be programmed to manage different parts without the need for physical reconfiguration. This adaptability makes them ideal for environments that require frequent changes in production. However, the initial investment and complexity of setup can be significant, often requiring specialized knowledge for maintenance and operation. Furthermore, while they excel in speed, their precision can vary based on the specific application and calibration.

Illustrative image related to medical parts feeding systems
How Do Manual Feeding Techniques Compare?
Manual feeding techniques involve human operators placing parts into machines or assembly lines. This method is often seen as a cost-effective solution, especially for small-scale operations or those producing highly customized components. One of the main advantages is the simplicity of implementation, as it requires minimal technology and can be quickly adjusted based on immediate needs. However, the reliance on human labor introduces variability in performance and can lead to higher error rates. Additionally, as production scales, labor costs may rise, making this approach less viable for larger operations.
Conclusion: How Should B2B Buyers Choose the Right Solution?
For B2B buyers in the medical manufacturing sector, selecting the appropriate parts handling solution requires a careful assessment of operational needs, budget constraints, and production goals. Medical parts feeding systems are ideal for high-volume, precision-oriented applications, while automated robotic systems offer unparalleled flexibility and speed for diverse production lines. On the other hand, manual feeding techniques may be suitable for niche applications with lower production demands. Ultimately, buyers should consider their long-term objectives, the complexity of their operations, and the total cost of ownership when making a decision. By aligning the chosen solution with specific production requirements, companies can enhance efficiency and maintain a competitive edge in the dynamic medical industry.
Essential Technical Properties and Trade Terminology for medical parts feeding systems
What Are the Key Technical Properties of Medical Parts Feeding Systems?
Understanding the essential technical specifications of medical parts feeding systems is vital for B2B buyers in the medical industry. Here are several critical properties to consider:

Illustrative image related to medical parts feeding systems
-
Material Grade
Medical parts feeders often utilize materials such as stainless steel or high-grade polymers to ensure durability and compliance with health regulations. Stainless steel is favored for its resistance to corrosion and ease of cleaning, making it suitable for sterile environments. Selecting the appropriate material grade is crucial for meeting industry standards and ensuring the longevity of the equipment. -
Tolerance
Tolerance refers to the allowable deviation from a specified dimension. In medical manufacturing, high tolerances (often within ±0.01 mm) are essential for ensuring that parts fit accurately and function as intended. This precision is vital to maintaining the safety and efficacy of medical devices, making it a key consideration for buyers. -
Feed Rate
The feed rate indicates how quickly parts are delivered to the assembly line, typically measured in parts per minute (PPM). An optimal feed rate ensures efficient production without bottlenecks. Buyers must assess their production demands and choose systems that can sustain the required throughput while maintaining accuracy. -
Cleanroom Compatibility
Many medical applications require operations to be conducted in cleanroom environments. Equipment designed for these settings must meet specific standards, such as ISO Class 5. Cleanroom compatibility ensures that the feeding systems do not introduce contaminants that could compromise product integrity, making this a critical specification for buyers. -
Flexibility and Modularity
With the rapidly changing landscape of medical manufacturing, the ability to adapt to new parts and processes is essential. Flexible and modular feeding systems can accommodate various component shapes and sizes, reducing the need for multiple machines. This adaptability can significantly lower operational costs and improve production efficiency.
What Are Common Trade Terms in Medical Parts Feeding Systems?
Familiarity with industry jargon is essential for effective communication and negotiation in the medical parts feeding systems market. Here are some key terms:
-
OEM (Original Equipment Manufacturer)
An OEM refers to a company that produces parts or equipment that may be marketed by another company. Understanding OEM relationships is important for buyers as it can affect lead times, costs, and the quality of the components being supplied. -
MOQ (Minimum Order Quantity)
MOQ is the smallest quantity of a product that a supplier is willing to sell. Knowing the MOQ helps buyers plan their procurement strategies and manage inventory effectively, especially in industries where demand can fluctuate. -
RFQ (Request for Quotation)
An RFQ is a document sent to suppliers to request pricing and terms for specific products. It is a crucial step in the procurement process, allowing buyers to compare offers and negotiate terms that meet their budget and project requirements. -
Incoterms (International Commercial Terms)
Incoterms are a set of predefined international trade terms that outline the responsibilities of buyers and sellers regarding shipping, insurance, and tariffs. Understanding these terms is vital for B2B transactions, particularly when sourcing equipment from international suppliers. -
Lead Time
Lead time refers to the time taken from placing an order to receiving the product. This is a critical factor for buyers in the medical industry, where timely delivery can significantly impact production schedules and regulatory compliance. -
HACCP (Hazard Analysis Critical Control Point)
HACCP is a systematic preventive approach to food safety that is also applicable in medical manufacturing. Understanding HACCP principles can help buyers ensure that their feeding systems meet safety and quality standards, particularly when dealing with products that may come into contact with patients.
By grasping these technical properties and trade terms, B2B buyers can make informed decisions when selecting medical parts feeding systems, ultimately enhancing their operational efficiency and compliance with industry standards.
Navigating Market Dynamics and Sourcing Trends in the medical parts feeding systems Sector
What Are the Key Market Dynamics and Trends in Medical Parts Feeding Systems?
The global medical parts feeding systems market is witnessing a significant transformation driven by technological advancements, regulatory changes, and the increasing demand for automation in manufacturing processes. International B2B buyers, particularly from regions such as Africa, South America, the Middle East, and Europe, are increasingly seeking suppliers that can provide solutions that are both efficient and adaptable. Key drivers include the rise of Industry 4.0, which emphasizes smart manufacturing and flexible automation. This shift necessitates the integration of innovative technologies such as IoT-enabled feeders and machine learning algorithms that enhance operational efficiency and predictive maintenance.
Emerging sourcing trends in the sector highlight the importance of modular and flexible feeding systems capable of handling diverse medical components. The demand for systems like FlexiBowl, which can adapt to various part sizes and shapes, is on the rise. Additionally, the need for precision and quality assurance is becoming paramount, with B2B buyers gravitating towards suppliers who can provide robust quality control measures integrated into their feeding systems. Moreover, as global supply chains become increasingly interconnected, buyers are looking for suppliers who can ensure timely delivery and responsiveness to market fluctuations.
How Are Sustainability and Ethical Sourcing Shaping the Medical Parts Feeding Systems Market?
Sustainability and ethical sourcing are becoming critical considerations for international B2B buyers in the medical parts feeding systems sector. The environmental impact of manufacturing processes is under scrutiny, leading companies to adopt greener practices. This includes utilizing sustainable materials, reducing waste, and minimizing energy consumption during production. Buyers are increasingly inclined to partner with suppliers that demonstrate a commitment to sustainability through certifications such as ISO 14001, which indicates effective environmental management systems.
Ethical supply chains are also gaining traction, as businesses recognize the importance of transparency and social responsibility in their sourcing practices. B2B buyers are looking for suppliers that adhere to ethical labor practices and demonstrate a commitment to social sustainability. This not only enhances brand reputation but also mitigates risks associated with supply chain disruptions. By choosing suppliers who prioritize sustainability and ethical sourcing, companies can contribute to a more responsible industry while also meeting regulatory requirements and consumer expectations.
What Is the Evolution of Medical Parts Feeding Systems in the B2B Landscape?
The evolution of medical parts feeding systems has been marked by a shift from traditional hard automation to more flexible and intelligent solutions. Initially, feeding systems were rigid, designed for specific tasks and requiring extensive downtime for changeovers. However, as the medical industry has evolved, so too have the requirements for production systems. The introduction of flexible feeders like the FlexiBowl has revolutionized the market, allowing manufacturers to handle a variety of components with minimal setup time.
This evolution reflects broader trends in the manufacturing sector, where agility and adaptability are paramount. The integration of advanced technologies, such as automation, artificial intelligence, and data analytics, has further enhanced the capabilities of medical parts feeding systems. As global demand for medical devices continues to rise, the focus on efficiency, flexibility, and sustainability in feeding systems will only intensify, creating significant opportunities for B2B buyers to optimize their production processes.
Frequently Asked Questions (FAQs) for B2B Buyers of medical parts feeding systems
-
How do I solve issues with part misalignment in medical feeding systems?
To address part misalignment, first, evaluate the feeder’s orientation mechanisms and ensure they are correctly calibrated for the specific parts being processed. Utilize advanced vision systems or sensors to detect misalignment and adjust the feeder’s settings accordingly. Regular maintenance and cleaning of the feeding system can also prevent debris from causing misalignment. Additionally, consider implementing flexible feeding solutions, like those offered by systems such as FlexiBowl®, which can adapt to various part shapes and sizes, enhancing overall accuracy. -
What is the best feeding system for handling delicate medical components?
For handling delicate medical components, a flexible feeder system, such as the FlexiBowl®, is highly recommended. This system is designed to accommodate a wide range of part sizes and shapes without causing damage. Its ability to adapt quickly between different components minimizes downtime and enhances efficiency. Look for systems that offer features like soft handling and precise orientation to ensure the integrity of sensitive items like syringes, vials, and other fragile components. -
How can I ensure compliance with international quality standards when sourcing medical parts feeding systems?
To ensure compliance with international quality standards, verify that the supplier’s feeding systems are certified according to relevant industry standards such as ISO 13485 for medical devices. Request documentation that demonstrates adherence to regulatory requirements, including quality management systems and cleanroom certifications. Engage in discussions with potential suppliers about their quality assurance processes, including testing and validation methods, to ensure their systems meet your specific needs and international standards. -
What are the typical lead times for custom medical parts feeding systems?
Lead times for custom medical parts feeding systems can vary significantly based on complexity and the supplier’s capacity. Generally, you can expect a timeframe of 6 to 12 weeks for design, manufacturing, and testing of custom systems. It’s crucial to communicate your specific requirements early in the process to avoid delays. Establish a clear timeline with your supplier and inquire about their production schedule to ensure timely delivery that aligns with your project needs. -
What factors should I consider when vetting suppliers for medical feeding systems?
When vetting suppliers, consider their experience in the medical industry, customer reviews, and case studies that highlight successful implementations. Evaluate their technical capabilities, including the flexibility of their feeding systems and their ability to customize solutions. Ensure they provide robust after-sales support and maintenance services. Additionally, assess their compliance with international regulations and certifications to guarantee quality and reliability in your supply chain. -
What are the minimum order quantities (MOQs) for medical parts feeding systems?
Minimum order quantities (MOQs) for medical parts feeding systems vary by supplier and product type. Some manufacturers may have low MOQs for standard systems, while custom solutions may require larger orders to justify production costs. It’s advisable to discuss your specific needs with potential suppliers and inquire about options for trial orders or smaller quantities, especially if you’re testing new systems or working within a limited budget. -
What payment terms are common for international B2B transactions in medical parts feeding systems?
Common payment terms for international B2B transactions typically include options such as net 30, net 60, or upfront payments for custom orders. Many suppliers also accept letters of credit or escrow services to secure transactions. Ensure to clarify payment terms before finalizing agreements and consider negotiating terms that align with your cash flow needs. Understanding currency exchange rates and potential transaction fees is also essential for international purchases. -
How do logistics and shipping impact the procurement of medical parts feeding systems?
Logistics and shipping play a critical role in the procurement process, particularly for international buyers. Factors such as shipping methods, customs regulations, and lead times can significantly affect delivery schedules. It’s vital to work with suppliers that have experience navigating international shipping and can provide guidance on logistics planning. Additionally, consider potential costs associated with shipping, insurance, and duties to ensure a clear understanding of the total procurement expenses.
Top 8 Medical Parts Feeding Systems Manufacturers & Suppliers List
1. Hoosier Feeder – Precision Feeders and Conveyors
Domain: hoosierfeeder.com
Registered: 2012 (13 years)
Introduction: Precision feeders and conveyors for handling pills, bottles, vials, and other sensitive medical components. Equipment designed for high standards in pharmaceutical and medical manufacturing, featuring stainless steel construction and sanitary design for cleanroom requirements. Includes vibratory feeders, stainless steel conveyors, and centrifugal feeders. Specific products mentioned: Scallop Centr…
2. FlexiBowl – Medical Parts Feeder
Domain: flexibowl.com
Registered: 2012 (13 years)
Introduction: FlexiBowl® is a medical parts feeder designed for flexibility and adaptability in manufacturing processes. Key features include:
– Certified for ISO Class 5 controlled environments (ISO 14644-1).
– Pneumatic components specific for cleanroom use.
– Back-lighted glass plate and perforated top screen to favor laminar flow.
– Available for FlexiBowl® 350/500/650/800 feeding systems.
– Product contact…
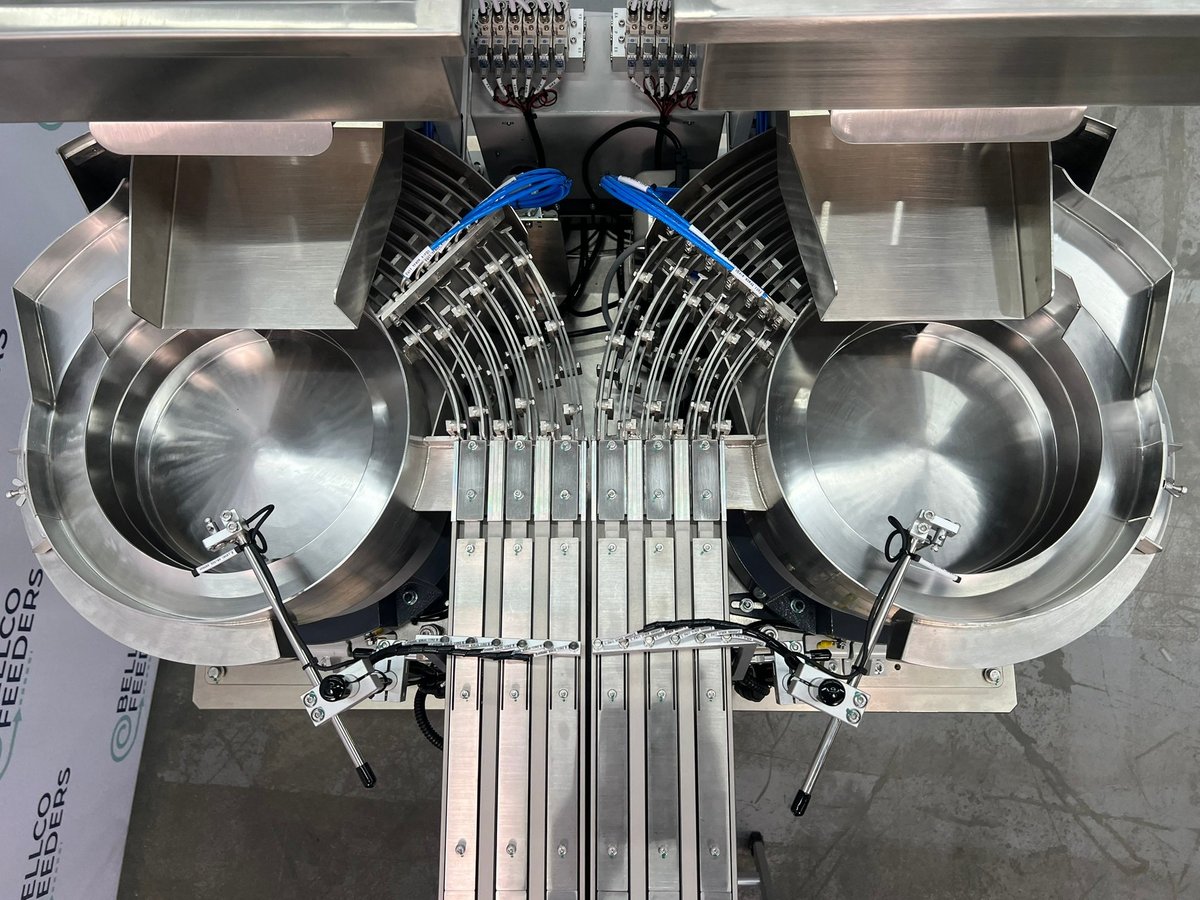
3. Bellco Feeders – Vibratory Parts Feeding Systems
Domain: bellcofeeders.com
Registered: 2022 (3 years)
Introduction: Bellco Feeders specializes in the design, development, and manufacturing of vibratory parts feeding systems. Key products include: 1. Vibratory Bowl Feeders – Custom-made to fit specific applications, known for quality and reliability. 2. STEP Feeders – Designed for efficient part handling. 3. Bellco Flex Feeders – Innovative feeding solutions tailored to unique geometries. 4. Bulk Part Hoppers – …
4. Performance Feeders – Vibratory Feeders
Domain: performancefeeders.com
Registered: 1999 (26 years)
Introduction: Performance Feeders offers a range of industrial automation products including vibratory feeders, centrifugal feeders, custom conveyor systems, and part handling systems. Key products include:
– Vibratory Feeders: Designed for gentle feeding of parts, including a new 3/16th green directional fiber mat for delicate applications.
– PFS3 Step Feeder: The largest in the step feeder line, featuring a ‘…
5. Fortville Feeders – Parts Feeding Systems
Domain: fortvillefeeders.com
Registered: 1997 (28 years)
Introduction: Fortville Feeders manufactures Parts Feeding Systems that are engineered for easy integration into automation systems. Key products include: Bowl Feeders, Flex Feeders, Escapements and Mechanisms, Tracking Systems, Sound Enclosures, Hoppers, Elevators, Gondolas, and Frames. They offer a Premium Five-Year Warranty, the longest in the industry, emphasizing maintenance and changeover friendliness. Th…
6. Vibratory Feeders – Pharmaceutical Bowl Parts Handling System
Domain: vibratoryfeeders.com
Registered: 1998 (27 years)
Introduction: Pharmaceutical Vibratory Feeder Bowl Parts Handling System; Key Features:
– Designed for pharmaceutical manufacturing, ensuring precision and compliance with FDA guidelines.
– Materials: Primarily 304 and 316L stainless steel; options for aluminum and nickel available.
– Components: Outside track bowl feeders and cascade bowl feeders recommended; supply hoppers for low to medium volume production…
7. Emerson – Feeding Solutions
Domain: emerson.com
Registered: 1995 (30 years)
Introduction: Feeding Solutions include Proportional Valves, Fuel Gas & Shutoff Valves, Sensors & Switches, and Handling Solutions such as Seat Valves.
8. Vibratory Feeders – Parts Feeding Solutions
Domain: vibratory-feeders.com
Registered: 2001 (24 years)
Introduction: Parts feeders are vibratory mechanisms that distribute parts for robots or automated processes. They receive equipment in bulk and mechanically manipulate them at a predetermined frequency for consistent feeding. Types of parts feeders include: 1. Centrifugal Feeders: High-speed operation, gentle handling of delicate parts, low noise, customizable for orientation, minimal maintenance. Applications…
Strategic Sourcing Conclusion and Outlook for medical parts feeding systems
In navigating the complex landscape of medical parts feeding systems, strategic sourcing emerges as a critical factor for international B2B buyers. The insights gleaned from industry leaders highlight the necessity of adopting flexible and efficient feeding solutions that not only meet stringent regulatory standards but also enhance production agility. Emphasizing technologies like FlexiBowl and advanced vibratory feeders can significantly reduce downtime and improve operational throughput, addressing the unique challenges faced in diverse markets across Africa, South America, the Middle East, and Europe.
Investing in high-quality, adaptable feeding systems ensures that manufacturers can swiftly respond to changing production demands and maintain competitive advantages. Furthermore, prioritizing suppliers with a proven track record in quality and service guarantees a smoother integration process, ultimately leading to enhanced product quality and reliability.

Illustrative image related to medical parts feeding systems
As the global medical sector continues to evolve, now is the time for B2B buyers to leverage these insights and invest strategically in their sourcing decisions. Embrace the future of manufacturing by exploring innovative feeding solutions that align with your operational goals. Reach out to leading suppliers today to discuss how you can enhance your production capabilities and stay ahead in this dynamic industry.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.

Illustrative image related to medical parts feeding systems
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.