Perineal Fenestrated Drape Explained: From A to Z for B2B Buyers
Introduction: Navigating the Global Market for perineal fenestrated drape
In the rapidly evolving healthcare landscape, sourcing high-quality perineal fenestrated drapes poses a significant challenge for international B2B buyers. These essential surgical tools are vital for ensuring patient safety and procedural efficiency, particularly in environments where infection control is paramount. This comprehensive guide delves into the multifaceted world of perineal fenestrated drapes, covering various types, applications, and critical considerations for supplier vetting.
With insights into cost structures, compliance standards, and emerging market trends, this resource equips buyers from Africa, South America, the Middle East, and Europe—such as those in Saudi Arabia and Brazil—with the knowledge needed to make informed purchasing decisions. By addressing the unique requirements of diverse healthcare systems and operational environments, this guide empowers businesses to navigate the complexities of the global market effectively.
Whether you are looking to enhance surgical outcomes or streamline procurement processes, understanding the nuances of perineal fenestrated drapes is crucial. This guide serves as a valuable tool, providing actionable insights and strategic advice tailored to the needs of B2B buyers, ultimately leading to improved patient care and operational excellence in surgical settings.
Understanding perineal fenestrated drape Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
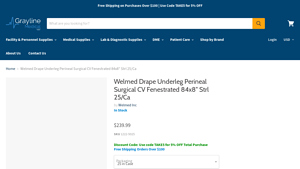
| Welmed Underleg Perineal Drape | Disposable, sterile, latex-free, 84×8 inches, bilateral fenestration | General surgical procedures | Pros: Cost-effective, easy to use. Cons: Non-returnable due to manufacturer restrictions. |
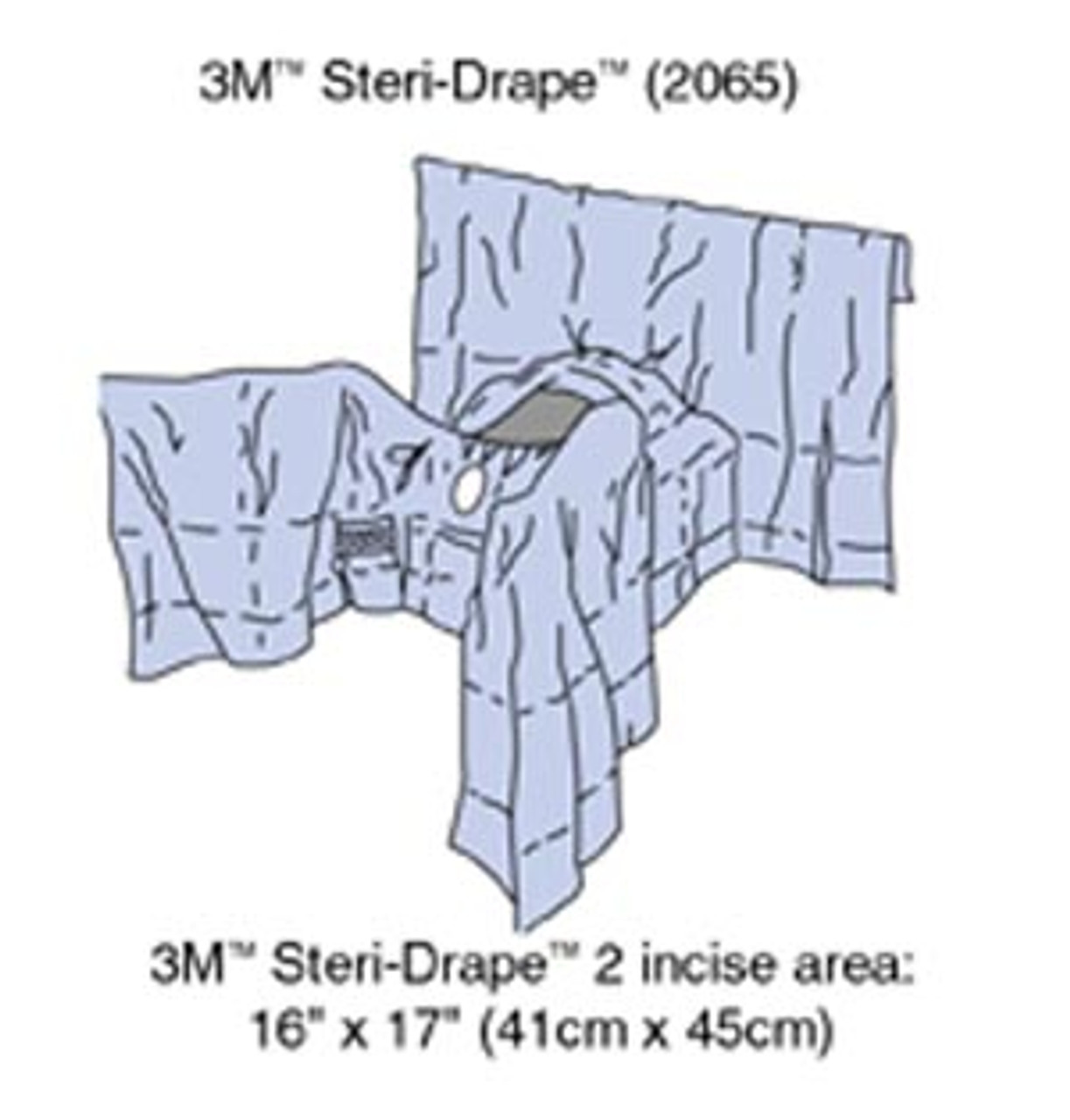
| HALYARD Universal Fenestrated Drape | Flame-resistant, low-lint fabric, alcohol-resistant, various fabric reinforcements | High-risk surgical environments | Pros: High safety standards, durable. Cons: Higher price point. |
| Winner Medical Laparotomy Drape | Designed for laparotomy, 2-3 layers, integrated instrument pouches | Abdominal surgeries | Pros: Flexibility in design, prevents infection. Cons: Limited to specific surgical types. |
| GRI Alleset Lithotomy T-Drape | Absorbent reinforcement, tube holders, large dimensions (60 x 31 x 75 in) | Urologic and gynecologic surgeries | Pros: Excellent fluid control, versatile. Cons: Bulkier, may require more storage space. |
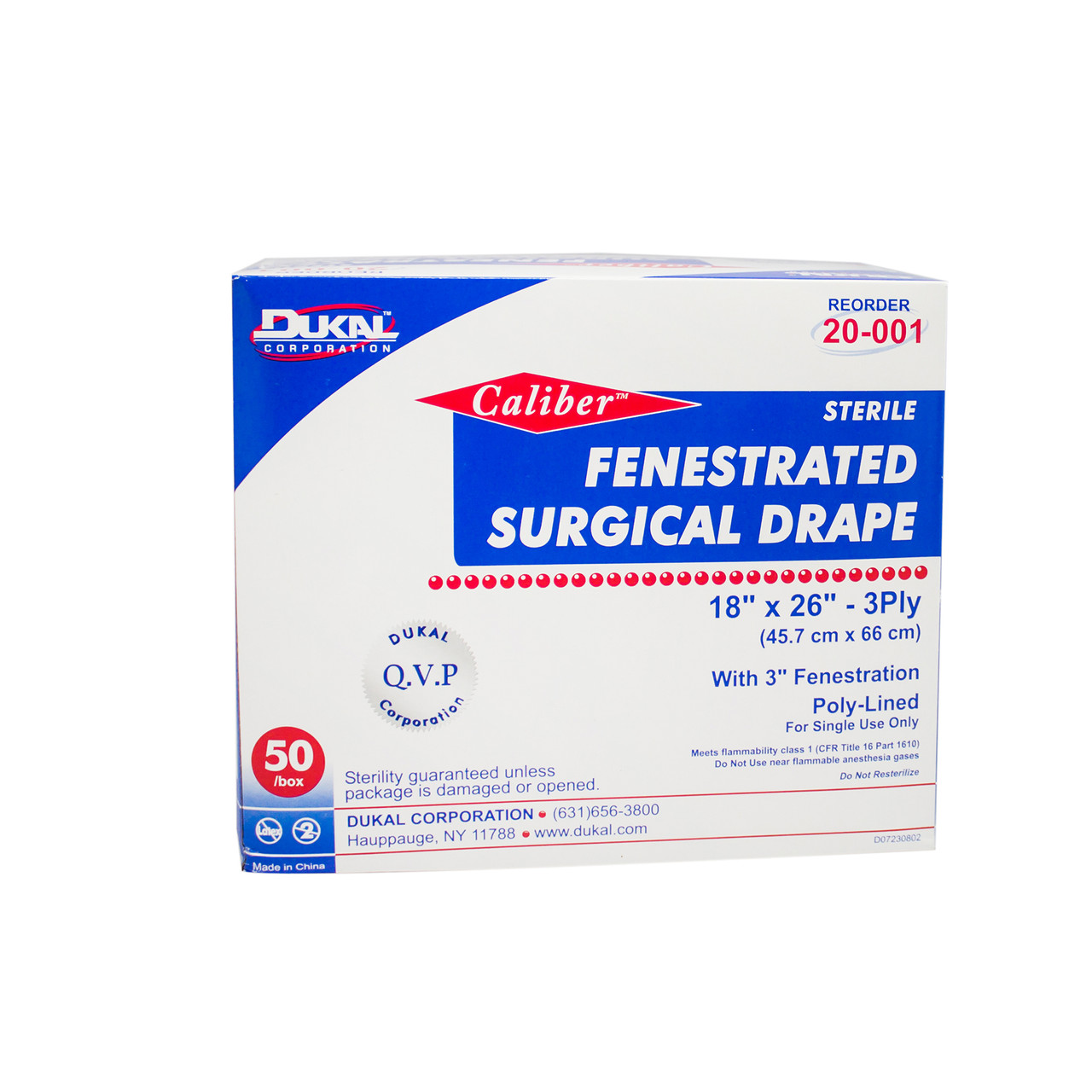
| Dukal Fenestrated Surgical Drape | Variety of sizes, disposable, designed to minimize contamination | General and specialty surgeries | Pros: Wide availability, easy disposal. Cons: May lack advanced features found in premium options. |
What are the Key Characteristics of the Welmed Underleg Perineal Drape?
The Welmed Underleg Perineal Drape is a disposable surgical drape that measures 84×8 inches and is designed for bilateral use. It is sterile and made without natural rubber latex, catering to patients with latex allergies. This drape is particularly suitable for general surgical procedures where rapid setup and ease of use are paramount. Buyers should consider its cost-effectiveness and the convenience of disposable products, though they must also note the non-returnable policy due to manufacturer restrictions.
How Does the HALYARD Universal Fenestrated Drape Stand Out?
The HALYARD Universal Fenestrated Drape is notable for its maximum flame resistance, making it ideal for use around lasers and electrical instruments. Its fabric is resistant to tearing and strikethrough from alcohol and other solutions, ensuring a secure sterile field. This drape is primarily utilized in high-risk surgical environments, where patient safety is critical. While it offers high durability and safety features, buyers should be prepared for a higher price point.
In What Situations is the Winner Medical Laparotomy Drape Most Effective?
The Winner Medical Laparotomy Drape is specifically designed for laparotomy surgeries, featuring a two to three-layer construction that provides a cloth-like feel while preventing infection transfer. Its design includes integrated instrument pouches, enhancing convenience during procedures. This drape is particularly suitable for abdominal surgeries, making it a preferred choice among surgical teams. Buyers should consider its flexibility and infection control features, although its specialized design may limit its applications.
What Benefits Does the GRI Alleset Lithotomy T-Drape Provide?
The GRI Alleset Lithotomy T-Drape is designed with absorbent reinforcement and tube holders, catering to urologic and gynecologic surgical needs. Its large dimensions (60 x 31 x 75 inches) provide ample coverage, making it versatile for various procedures. This drape excels in fluid management, ensuring a clean operative field. While it offers excellent functionality, buyers should be aware of its bulkier size, which may necessitate additional storage solutions.
Why Choose Dukal Fenestrated Surgical Drapes?
Dukal Fenestrated Surgical Drapes are disposable options available in various sizes, designed to minimize contamination during procedures. They are suitable for general and specialty surgeries, providing a straightforward solution for healthcare facilities. Their wide availability and easy disposal make them attractive to many buyers. However, buyers should be mindful that these drapes may lack some advanced features found in higher-end alternatives, which could be a consideration for specialized surgical environments.
Key Industrial Applications of perineal fenestrated drape
| Industry/Sector | Specific Application of Perineal Fenestrated Drape | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Healthcare Facilities | Surgical procedures in gynecology and urology | Enhances patient safety and infection control during surgeries | Ensure compliance with local regulations and sterility standards |
| Veterinary Clinics | Surgical interventions for animal care | Provides a sterile field, reducing the risk of post-operative infections | Assess the drape’s size and material compatibility with animal types |
| Emergency Medical Services | Rapid response for trauma care in pre-hospital settings | Facilitates quick and safe intervention for patients in emergencies | Look for lightweight, compact options for easy transport and storage |
| Research Institutions | Use in clinical trials involving surgical interventions | Supports sterile environments essential for the integrity of research | Prioritize high-quality materials that meet specific research protocols |
| Cosmetic Surgery Centers | Aesthetic surgical procedures requiring precision draping | Improves operational efficiency and minimizes contamination risks | Evaluate product availability and supplier reliability for consistent supply |
How is the Perineal Fenestrated Drape Utilized in Healthcare Facilities?
In healthcare facilities, perineal fenestrated drapes are primarily employed during surgical procedures in gynecology and urology. These drapes create a sterile field, which is crucial for minimizing infection risks during operations. By providing a barrier against contaminants, they enhance patient safety, allowing healthcare professionals to focus on the procedure without worrying about potential infections. For international buyers, particularly in regions like Africa and the Middle East, it is vital to ensure that the drapes meet local healthcare regulations and sterility standards to maintain operational integrity.
What Role Does the Perineal Fenestrated Drape Play in Veterinary Clinics?
In veterinary clinics, perineal fenestrated drapes are used during surgical interventions for animals, such as spaying or neutering. These drapes ensure a sterile environment, thereby reducing the likelihood of post-operative infections, which can significantly impact recovery time and overall animal health. Buyers in this sector should consider the size and material of the drapes, as compatibility with different animal types is essential for effective use.
Illustrative image related to perineal fenestrated drape
How Are Perineal Fenestrated Drapes Beneficial for Emergency Medical Services?
Emergency medical services utilize perineal fenestrated drapes in trauma care settings to provide rapid and sterile environments for patients requiring immediate attention. These drapes facilitate quick interventions while ensuring that the risk of contamination is minimized. For B2B buyers, sourcing lightweight and compact options is crucial, as these features enhance portability and storage, making them ideal for emergency situations.
Why Are Perineal Fenestrated Drapes Important in Research Institutions?
Research institutions frequently employ perineal fenestrated drapes during clinical trials that involve surgical interventions. These drapes help maintain sterile conditions, which are critical for the accuracy and integrity of research outcomes. Buyers in this sector should prioritize high-quality materials that comply with specific research protocols, ensuring that the drapes do not compromise the study’s validity.
How Do Cosmetic Surgery Centers Benefit from Using Perineal Fenestrated Drapes?
Cosmetic surgery centers utilize perineal fenestrated drapes to enhance operational efficiency and minimize contamination risks during aesthetic procedures. These drapes provide a secure, sterile field that allows surgeons to perform with precision. When sourcing these drapes, it is essential for buyers to evaluate product availability and supplier reliability to ensure a consistent supply, which is vital for maintaining patient care standards.
Illustrative image related to perineal fenestrated drape
3 Common User Pain Points for ‘perineal fenestrated drape’ & Their Solutions
Scenario 1: Inadequate Sterility and Infection Control Challenges
The Problem: In the healthcare industry, the importance of maintaining a sterile environment during surgical procedures cannot be overstated. B2B buyers often face challenges in ensuring that the perineal fenestrated drapes they procure meet the highest sterility standards. Non-compliance with sterilization protocols can lead to increased infection rates, ultimately affecting patient outcomes and hospital reputations. Buyers may also struggle with the difficulty of verifying the sterility of drapes, especially when sourcing from different suppliers across regions.
The Solution: To mitigate the risk of infection, it is crucial for buyers to establish strict sourcing protocols for perineal fenestrated drapes. Opt for suppliers who provide comprehensive documentation of sterilization processes, including certifications that confirm compliance with industry standards such as ISO 13485. Conduct audits or request samples from suppliers to verify their sterilization methods and quality control measures. Additionally, consider drapes that are individually packaged and labeled with clear expiration dates to ensure freshness and sterility. Implementing a robust inventory management system can help track the usage and replacement schedules for these drapes, ensuring that only the most current, sterile products are used during procedures.
Scenario 2: Difficulty in Customization and Fit for Specific Procedures
The Problem: Different surgical procedures may require unique draping solutions, particularly for perineal surgeries that often need specific fenestration sizes and placements. B2B buyers frequently encounter the challenge of finding drapes that not only fit the anatomical requirements of their patients but also conform to the diverse surgical techniques used by their medical staff. This can lead to operational inefficiencies and increased costs if the wrong drapes are purchased.
The Solution: Buyers should prioritize suppliers that offer customizable perineal fenestrated drapes tailored to specific surgical needs. Engage in discussions with manufacturers about their customization capabilities, including the possibility of creating drapes with unique fenestration sizes and placement options. Collaborate with surgical teams to understand their requirements better and provide feedback to suppliers. Additionally, consider creating a standardization protocol within your facility that outlines the specific drapes needed for common procedures. By establishing a partnership with a reliable supplier that allows for customization, you can ensure that your surgical teams have the right tools at their disposal, thereby improving efficiency and patient care.
Scenario 3: Managing Costs While Ensuring Quality
The Problem: With tight budgets and the constant pressure to reduce operational costs, B2B buyers in healthcare often struggle to find a balance between cost-effectiveness and quality when purchasing perineal fenestrated drapes. They may be tempted to opt for lower-cost options that compromise on quality, leading to potential increases in complications, re-surgery rates, and overall dissatisfaction among healthcare professionals.
The Solution: To navigate the cost-quality dilemma, buyers should conduct a thorough cost-benefit analysis when selecting perineal fenestrated drapes. Look for suppliers that offer volume discounts or bundled purchasing options, which can significantly reduce costs without sacrificing quality. Additionally, consider investing in high-quality drapes that may have a higher upfront cost but ultimately reduce the risk of complications and associated costs in the long term. Establishing a vendor evaluation process that includes criteria such as product quality, supplier reliability, and after-sales support can help ensure that you are making informed purchasing decisions. Leveraging group purchasing organizations (GPOs) can also provide access to negotiated pricing without compromising on the quality of medical supplies.
Strategic Material Selection Guide for perineal fenestrated drape
What Are the Common Materials Used in Perineal Fenestrated Drapes?
When selecting materials for perineal fenestrated drapes, it is essential to consider their properties, advantages, and limitations. Below, we analyze four common materials used in the manufacturing of these drapes, highlighting their suitability for various applications.

Illustrative image related to perineal fenestrated drape
1. Non-Woven Fabric
Key Properties: Non-woven fabrics are composed of fibers bonded together through mechanical, thermal, or chemical processes. They offer excellent fluid resistance and breathability, making them suitable for surgical applications.
Pros & Cons: Non-woven fabrics are lightweight, cost-effective, and disposable, which enhances convenience in surgical settings. However, they may not provide the same level of durability as woven fabrics, potentially limiting their use in longer procedures.
Impact on Application: These fabrics are compatible with various sterilization methods, including ethylene oxide and gamma radiation. Their fluid resistance is critical in preventing contamination during procedures.
Considerations for International Buyers: Buyers should ensure compliance with international standards such as ASTM for medical textiles. In regions like Africa and South America, where healthcare infrastructure may vary, the availability of non-woven materials can be a significant advantage due to their cost-effectiveness.

Illustrative image related to perineal fenestrated drape
2. Laminated Fabric
Key Properties: Laminated fabrics are constructed by bonding a layer of polymer film to a textile substrate. This combination provides enhanced barrier properties against fluids and microorganisms.
Pros & Cons: The primary advantage of laminated fabrics is their superior fluid resistance and durability, making them suitable for high-risk surgical environments. However, they can be more expensive and may require specialized manufacturing processes.
Impact on Application: Laminated fabrics are particularly effective in surgeries where fluid management is critical, such as urological or gynecological procedures. Their compatibility with various sterilization methods also enhances their utility.
Considerations for International Buyers: Buyers in the Middle East and Europe may prefer laminated fabrics due to stringent regulations regarding infection control. Ensuring that these materials meet local compliance standards is crucial for successful procurement.
3. SMS (Spunbond-Meltblown-Spunbond) Fabric
Key Properties: SMS fabric combines spunbond and meltblown layers, offering excellent barrier protection while maintaining breathability. It is known for its strength and softness.
Pros & Cons: SMS fabrics provide a balance between comfort and protection, making them ideal for prolonged use. However, they can be more costly than basic non-woven options and may not be as readily available in all markets.
Impact on Application: The multilayer structure of SMS fabric makes it suitable for high-fluid environments, reducing the risk of strike-through. This is particularly important in perineal surgeries where fluid management is critical.

Illustrative image related to perineal fenestrated drape
Considerations for International Buyers: In regions like Brazil and Saudi Arabia, where healthcare facilities are increasingly adopting advanced technologies, SMS fabrics may be favored for their performance. Compliance with local standards, such as those set by ANVISA in Brazil, should be verified.
4. Polyester Fabric
Key Properties: Polyester is a synthetic fabric known for its strength, durability, and resistance to wrinkling and shrinking. It can be treated to enhance its barrier properties.
Pros & Cons: Polyester drapes are reusable, which can lead to cost savings over time. However, their higher initial investment and the need for proper cleaning and sterilization protocols can be seen as disadvantages.
Impact on Application: Polyester drapes are suitable for various surgical procedures, including those requiring multiple uses. Their durability makes them a preferred choice for facilities with high patient turnover.

Illustrative image related to perineal fenestrated drape
Considerations for International Buyers: In Europe, where sustainability is increasingly prioritized, reusable polyester drapes may align with environmental goals. Buyers should ensure that these products meet the necessary sterilization standards to maintain patient safety.
Summary Table
| Material | Typical Use Case for perineal fenestrated drape | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Non-Woven Fabric | General surgical procedures | Lightweight and cost-effective | Limited durability for long procedures | Low |
| Laminated Fabric | High-risk surgeries (urological, gynecological) | Superior fluid resistance | Higher cost and complex manufacturing | High |
| SMS Fabric | High-fluid environments | Comfort and excellent barrier protection | More expensive than basic options | Med |
| Polyester Fabric | Reusable surgical drapes | Durability and cost savings over time | Higher initial investment required | Med |
This analysis provides B2B buyers with a comprehensive understanding of the materials available for perineal fenestrated drapes, enabling informed purchasing decisions based on performance, cost, and compliance considerations.
In-depth Look: Manufacturing Processes and Quality Assurance for perineal fenestrated drape
What Are the Key Stages in the Manufacturing Process of Perineal Fenestrated Drapes?
Manufacturing perineal fenestrated drapes involves several critical stages that ensure high-quality production tailored for surgical environments. Understanding these stages helps B2B buyers gauge the reliability and efficiency of potential suppliers.

Illustrative image related to perineal fenestrated drape
1. Material Preparation
The first step in the manufacturing process is the careful selection and preparation of materials. Perineal fenestrated drapes are typically made from nonwoven fabrics, which provide a balance of softness, durability, and fluid resistance. Manufacturers often use SMS (Spunbond-Meltblown-Spunbond) or laminated fabrics to enhance barrier properties against fluids and pathogens.
In this stage, materials are sourced from certified suppliers, ensuring compliance with relevant health and safety standards. The materials undergo various pre-treatment processes, including sterilization, to eliminate any microbial contamination before production begins.
2. Forming the Drapes
Once the materials are prepared, the next stage involves cutting them into appropriate sizes and shapes. The fenestration—an opening designed for surgical access—is a critical design feature of these drapes. Advanced cutting techniques, such as die-cutting or laser cutting, are employed to ensure precision and consistency in fenestration dimensions.
During this stage, manufacturers also consider ergonomics and usability, ensuring that the drapes facilitate ease of handling and application in surgical settings.
3. Assembly
After forming, the various components of the drapes are assembled. This includes attaching reinforcement layers and any additional features, such as adhesive strips or tube holders. The assembly process is usually automated, but skilled operators oversee quality control to ensure every drape meets design specifications.
This stage may also involve the integration of features that enhance usability, such as quick-release tabs or adjustable straps, which are crucial in fast-paced surgical environments.

Illustrative image related to perineal fenestrated drape
4. Finishing
The finishing stage focuses on final touches that enhance the product’s quality and usability. This may include folding, packaging, and labeling the drapes. Quality checks are performed to ensure that the packaging is intact and sterile, ready for distribution.
Additionally, this stage often incorporates tests for fluid resistance, tensile strength, and overall durability to ensure that the drapes will perform as expected during surgical procedures.
How Is Quality Assurance Implemented in the Manufacturing of Perineal Fenestrated Drapes?
Quality assurance (QA) is a critical aspect of the manufacturing process, ensuring that perineal fenestrated drapes meet international standards and customer expectations. For B2B buyers, understanding the QA protocols can provide insights into the reliability of their suppliers.
Relevant International Standards for Quality Assurance
Manufacturers of perineal fenestrated drapes typically adhere to several international standards, including:
- ISO 9001: This standard ensures that organizations have a systematic approach to managing quality. It focuses on continuous improvement and customer satisfaction.
- CE Marking: For products sold in Europe, CE marking indicates conformity with health, safety, and environmental protection standards.
- API Standards: In some regions, specific quality protocols set by the American Petroleum Institute may apply, particularly for materials used in medical applications.
These certifications not only signify compliance but also instill confidence in B2B buyers regarding product reliability.

Illustrative image related to perineal fenestrated drape
Key Quality Control Checkpoints
To maintain high-quality standards, manufacturers implement multiple checkpoints throughout the production process:
- Incoming Quality Control (IQC): At this stage, raw materials are inspected upon arrival for compliance with specifications. This includes checking material properties and certifications.
- In-Process Quality Control (IPQC): During manufacturing, random samples are taken to ensure that production processes meet quality standards. This helps catch any deviations early in the production cycle.
- Final Quality Control (FQC): Once the drapes are completed, a final inspection is conducted. This includes checking for defects, ensuring proper fenestration, and verifying that packaging is sterile and intact.
Common Testing Methods for Quality Assurance
Manufacturers employ various testing methods to validate the quality of perineal fenestrated drapes, including:
- Microbial Barrier Testing: This test measures the drape’s ability to prevent microbial penetration, ensuring it meets safety standards.
- Fluid Resistance Testing: This assesses how well the drape can resist fluid penetration, a critical factor during surgical procedures.
- Tensile Strength Testing: This evaluates the durability of the drape under stress, ensuring it won’t tear during use.
How Can B2B Buyers Verify Supplier Quality Control?
To ensure that their suppliers adhere to stringent quality standards, B2B buyers should consider implementing several verification strategies:
Supplier Audits
Conducting regular audits of suppliers can help verify their compliance with quality standards. During an audit, buyers can assess manufacturing processes, quality control measures, and adherence to international standards. This proactive approach helps identify potential issues before they impact product quality.

Illustrative image related to perineal fenestrated drape
Requesting Quality Reports
Buyers should request comprehensive quality reports from suppliers. These reports should detail the results of various quality control tests, including compliance with international standards. Transparency in reporting helps build trust and enables buyers to make informed decisions.
Third-Party Inspections
Engaging third-party inspection agencies can provide an unbiased assessment of a supplier’s quality control processes. These agencies can conduct thorough inspections and testing, ensuring that the products meet the necessary safety and quality standards.
What Are the Quality Control Nuances for International B2B Buyers?
For B2B buyers operating in diverse regions such as Africa, South America, the Middle East, and Europe, understanding the nuances of quality control is essential:
- Regional Standards Compliance: Different countries may have specific regulatory requirements. Buyers should ensure that suppliers comply with local regulations in their target markets.
- Cultural Considerations: Understanding cultural differences in business practices can impact negotiations and expectations regarding quality assurance. Building relationships based on trust and transparency can enhance collaboration.
- Logistical Considerations: Buyers should consider the logistics of sourcing materials and products. Understanding the supply chain, including transportation and storage conditions, can influence product quality upon delivery.
By navigating these nuances effectively, international B2B buyers can establish fruitful partnerships with suppliers of perineal fenestrated drapes, ensuring high-quality products that meet their operational needs.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘perineal fenestrated drape’
To assist B2B buyers in sourcing perineal fenestrated drapes effectively, this guide outlines essential steps to ensure informed and strategic procurement. These drapes are critical for surgical procedures, and understanding the specific requirements and supplier capabilities will enhance operational efficiency and patient safety.
Step 1: Define Your Technical Specifications
Establishing clear technical specifications is the foundation of your sourcing process. Consider the dimensions, material composition (e.g., non-woven, SMS), and specific features like fenestration size and placement. This clarity will not only streamline your search but also help suppliers understand your exact needs.
- Material Requirements: Opt for drapes that are latex-free to minimize allergic reactions.
- Reinforcement Needs: Determine if you require additional reinforcement to prevent fluid leakage during procedures.
Step 2: Identify Regulatory Compliance Needs
Understanding the regulatory landscape is crucial, especially in diverse markets like Africa, South America, and the Middle East. Ensure that the drapes comply with local and international standards, such as ISO certifications or CE marking.
- Safety Standards: Look for drapes with flame resistance ratings if they will be used around lasers.
- Quality Assurance: Request documentation proving compliance with health regulations.
Step 3: Evaluate Potential Suppliers
Before committing to a supplier, conduct a thorough evaluation. Assess their market reputation, product quality, and customer service.
- Company Background: Request company profiles and certifications to verify their credibility.
- Client Testimonials: Seek references or case studies from buyers in similar regions to gauge reliability and performance.
Step 4: Request Samples for Testing
Obtaining samples is an essential step in the procurement process. Testing samples allows you to assess the quality, usability, and compatibility of the drapes with your surgical procedures.

Illustrative image related to perineal fenestrated drape
- Material Assessment: Evaluate the texture and thickness to ensure it meets your operational requirements.
- Functional Testing: Check for ease of application and secure attachment for lines and tubes.
Step 5: Compare Pricing and Terms
Once you have shortlisted potential suppliers, compare their pricing structures and terms. Look beyond just the price per unit; consider bulk purchase discounts and shipping costs.
- Total Cost of Ownership: Factor in potential hidden costs, such as shipping fees and import duties, especially for international suppliers.
- Payment Terms: Ensure the payment terms align with your budget cycles and financial strategies.
Step 6: Confirm Delivery and Inventory Management
Effective inventory management is crucial for maintaining operational continuity. Discuss delivery timelines and stock availability with your chosen supplier.
- Lead Time: Confirm the average processing time for orders to avoid delays in surgical schedules.
- Stock Levels: Ensure the supplier can maintain adequate inventory levels to meet your demands, especially in peak periods.
Step 7: Establish a Communication Plan
Once you’ve selected a supplier, establish a clear communication plan to facilitate ongoing collaboration. Regular communication ensures that any issues are addressed promptly and that you remain updated on product changes or new offerings.

Illustrative image related to perineal fenestrated drape
- Regular Check-ins: Schedule periodic reviews to discuss performance and any potential adjustments needed.
- Feedback Mechanism: Create a system for providing feedback on product performance and supplier service.
By following this checklist, B2B buyers can effectively source perineal fenestrated drapes, ensuring quality and compliance while optimizing operational efficiencies in healthcare settings.
Comprehensive Cost and Pricing Analysis for perineal fenestrated drape Sourcing
What Are the Key Cost Components for Perineal Fenestrated Drapes?
Understanding the cost structure of perineal fenestrated drapes is crucial for B2B buyers. The primary components include:
-
Materials: The choice of fabric (e.g., SMS, laminated) significantly affects the cost. Higher-quality, non-woven materials that offer better fluid resistance and lower lint production tend to be more expensive.
-
Labor: Labor costs encompass the wages paid to workers involved in manufacturing. Depending on the region, labor rates can vary significantly, influencing the overall price of the drapes.
-
Manufacturing Overhead: This includes costs related to utilities, maintenance, and other operational expenses that are necessary to keep manufacturing facilities running efficiently.
-
Tooling: Investment in specialized machinery and tools for producing drapes can add to the initial costs. The complexity of the design, such as fenestration patterns, may require advanced tooling.
-
Quality Control (QC): Ensuring that each batch of drapes meets safety and quality standards incurs additional costs, particularly for drapes intended for surgical use.
-
Logistics: Transporting products from manufacturers to international markets involves shipping costs, tariffs, and customs fees, which can vary widely based on location and shipping terms.
-
Margin: The supplier’s profit margin must also be considered, as it can vary based on competition, brand reputation, and market demand.
How Do Price Influencers Impact the Cost of Perineal Fenestrated Drapes?
Several factors can influence the pricing of perineal fenestrated drapes:
-
Volume/MOQ (Minimum Order Quantity): Suppliers often provide discounts for bulk purchases. Understanding MOQ can help buyers negotiate better pricing.
-
Specifications and Customization: Custom features, such as specific fenestration sizes or reinforced areas, can increase costs. Buyers should weigh the benefits of customization against the added expense.
-
Material Quality and Certifications: Drapes that meet higher safety and quality certifications, such as those compliant with ISO or FDA standards, may come at a premium.
-
Supplier Factors: The supplier’s reputation, reliability, and manufacturing capabilities can affect pricing. Established suppliers may charge more due to perceived quality and service levels.
-
Incoterms: The chosen Incoterms can impact the total cost of ownership. Buyers should consider terms like FOB (Free on Board) or CIF (Cost, Insurance, and Freight) to understand their responsibilities for shipping and insurance costs.
What Negotiation Strategies Can Help Buyers Optimize Costs?
B2B buyers should adopt several strategies to enhance cost-efficiency when sourcing perineal fenestrated drapes:
-
Leverage Volume Discounts: Consolidating orders or collaborating with other buyers can increase purchasing power, leading to better pricing.
-
Understand Total Cost of Ownership (TCO): Evaluate not just the purchase price but also associated costs such as shipping, storage, and potential waste from over-ordering.
-
Build Relationships with Suppliers: Establishing long-term relationships can lead to better terms, exclusive discounts, and improved service.
-
Request Quotes from Multiple Suppliers: Gathering competitive quotes can provide leverage in negotiations. It’s essential to compare not only prices but also the value offered in terms of quality and service.
-
Be Aware of Pricing Nuances in International Markets: Buyers from regions such as Africa, South America, the Middle East, and Europe should consider local market conditions, currency fluctuations, and import regulations that might impact pricing.
What Should Buyers Keep in Mind About Pricing for Perineal Fenestrated Drapes?
While indicative prices for perineal fenestrated drapes can range significantly based on the factors mentioned, it is essential for buyers to approach pricing with a comprehensive understanding. Factors such as market demand, regional availability, and specific product requirements can lead to variances in costs. Always ensure that you are aware of the total cost implications before finalizing any procurement decision, as this will help you make informed and cost-effective choices.
Alternatives Analysis: Comparing perineal fenestrated drape With Other Solutions
Understanding Alternatives to Perineal Fenestrated Drapes
In the medical field, the choice of surgical drapes is crucial for ensuring patient safety and procedural efficiency. While perineal fenestrated drapes are a popular option for specific surgeries, various alternatives may offer comparable or enhanced benefits. Evaluating these alternatives can help healthcare facilities make informed purchasing decisions that align with their operational needs and budgets.
Comparison Table
| Comparison Aspect | Perineal Fenestrated Drape | HALYARD* Universal Fenestrated Drapes | Winner Medical Laparotomy Drape |
|---|---|---|---|
| Performance | High fluid resistance, designed for perineal access | Excellent flame resistance, low lint | Effective barrier against infection |
| Cost | $239.99 per case (25 units) | Varies, generally higher than basic options | Competitive pricing, bulk discounts available |
| Ease of Implementation | Quick setup, disposable | Easy to use, requires minimal training | Streamlined setup, user-friendly design |
| Maintenance | Disposable, no maintenance required | Disposable, minimal waste management | Disposable, efficient for single use |
| Best Use Case | Gynecological and urological surgeries | General surgical procedures with laser use | Abdominal surgeries requiring perineal access |
Detailed Breakdown of Alternatives
HALYARD* Universal Fenestrated Drapes
HALYARD* drapes are engineered for versatility and safety, featuring high flame resistance, making them suitable for use around lasers and electrical instruments. Their fabric is designed to resist tearing and strikethrough, ensuring that fluids do not compromise sterility. However, these drapes may come at a higher cost, which could be a consideration for budget-conscious facilities. Their low linting property minimizes airborne bacteria transmission, making them a good choice for high-risk environments.
Winner Medical Laparotomy Drape
Winner Medical offers a laparotomy drape that is particularly beneficial for surgeries involving the abdomen. These drapes are designed with fenestrated openings to allow for sterile access while minimizing the risk of infection. The product is made from SMS or laminated fabric, providing a cloth-like feel that enhances patient comfort. While they are competitively priced, the drapes are best suited for specific surgical procedures, which may limit their use in more general applications.
Conclusion: How to Choose the Right Surgical Drape for Your Needs
When selecting a surgical drape, B2B buyers should consider not only the immediate costs but also the performance characteristics and specific use cases of each product. Perineal fenestrated drapes are excellent for targeted procedures, but alternatives like HALYARD* and Winner Medical options provide unique advantages that may better suit certain surgical environments. Evaluating the balance between cost, performance, and ease of use is essential for making the right choice that meets both clinical and budgetary requirements. Buyers should also consider their specific procedural needs, the types of surgeries performed, and the level of patient care desired when making their selection.
Essential Technical Properties and Trade Terminology for perineal fenestrated drape
What Are the Key Technical Properties of Perineal Fenestrated Drapes?
Understanding the essential technical properties of perineal fenestrated drapes is crucial for B2B buyers, especially in healthcare settings. Here are several critical specifications:

Illustrative image related to perineal fenestrated drape
-
Material Composition
Perineal fenestrated drapes are typically made from non-woven fabrics such as SMS (spunbond-meltblown-spunbond) or laminated materials. These materials offer superior barrier protection against fluid penetration and microbial transfer, ensuring a sterile environment during surgical procedures. Buyers should consider the material’s durability and resistance to tearing, which can impact the drape’s effectiveness in a high-stress operating room environment. -
Dimensions and Design
Standard dimensions for perineal fenestrated drapes usually measure around 84×8 inches, with fenestration sizes varying to accommodate different surgical needs. The design must facilitate ease of use, allowing for quick application while maintaining patient safety and comfort. Precise dimensions ensure that the drape fits securely and covers the required area, minimizing exposure to contaminants. -
Fenestration Size and Location
Fenestration refers to the openings designed in the drape to allow access to the surgical site. The size and placement of fenestrations are critical for procedural success, as they must align correctly with the surgical area. A well-placed fenestration reduces the risk of contamination and enhances the surgeon’s access, thereby improving surgical efficiency. -
Sterility Assurance
Most perineal fenestrated drapes are provided sterile and packaged to maintain sterility until use. This is crucial in preventing surgical site infections (SSIs). Buyers should verify that the products meet relevant sterilization standards and are labeled accordingly, ensuring compliance with healthcare regulations in their regions. -
Reinforcement Features
Many drapes come with reinforced sections, which are designed to handle the weight and movement of surgical instruments. Reinforcements can prevent slippage and absorb fluids, further protecting the sterile field. Understanding the specifics of these reinforcements can help buyers select the right product based on their procedural requirements.
What Are Common Trade Terms Related to Perineal Fenestrated Drapes?
Navigating the B2B landscape requires familiarity with industry jargon. Here are several common terms that buyers should know:
-
OEM (Original Equipment Manufacturer)
This term refers to a company that produces parts or equipment that may be marketed by another manufacturer. Understanding OEM relationships can help buyers identify quality products and the manufacturers behind them, ensuring they are sourcing from reputable suppliers. -
MOQ (Minimum Order Quantity)
MOQ is the smallest quantity of a product that a supplier is willing to sell. For perineal fenestrated drapes, MOQs can vary significantly among suppliers. Buyers should evaluate their needs against MOQs to avoid over-purchasing or facing stock shortages. -
RFQ (Request for Quotation)
An RFQ is a standard business process where buyers request pricing and terms from suppliers. When seeking perineal fenestrated drapes, issuing an RFQ allows buyers to compare options and negotiate better terms, ensuring they make informed purchasing decisions. -
Incoterms (International Commercial Terms)
These terms define the responsibilities of buyers and sellers in international transactions, clarifying who is responsible for shipping, insurance, and tariffs. Familiarity with Incoterms is essential for B2B buyers, especially when sourcing products globally. -
Lead Time
Lead time is the period from the placement of an order to its delivery. Understanding lead times for perineal fenestrated drapes is vital for inventory management, particularly in healthcare settings where timely access to surgical supplies can impact patient outcomes.
By grasping these technical properties and industry terms, B2B buyers can make more informed decisions when sourcing perineal fenestrated drapes, ultimately enhancing their procurement processes and operational efficiency.
Navigating Market Dynamics and Sourcing Trends in the perineal fenestrated drape Sector
What Are the Key Trends Driving the Perineal Fenestrated Drape Market?
The global market for perineal fenestrated drapes is shaped by several dynamic factors that are critical for B2B buyers, particularly in emerging markets such as Africa, South America, the Middle East, and Europe. A significant driver is the increasing emphasis on infection control and patient safety, which has led to heightened demand for high-quality, sterile surgical drapes. As hospitals and surgical centers prioritize patient outcomes, the adoption of advanced materials that minimize lint, absorb fluids, and resist strikethrough is becoming essential.
Emerging technologies are also influencing sourcing trends in this sector. For instance, the integration of digital platforms for procurement and inventory management is streamlining the supply chain process, allowing buyers to make informed decisions quickly. Additionally, manufacturers are focusing on innovation, developing drapes with enhanced features such as adhesive properties and reinforced fabrics to prevent slippage during procedures. This trend is particularly relevant for international buyers who seek reliable products tailored to various surgical needs.
Moreover, regulatory frameworks across different regions are evolving, necessitating compliance with stringent safety and quality standards. Buyers are increasingly seeking suppliers who can demonstrate adherence to these regulations, which adds another layer of complexity to sourcing. As a result, understanding local market dynamics, including pricing strategies and distribution channels, is crucial for successful procurement.
How Is Sustainability and Ethical Sourcing Impacting the Perineal Fenestrated Drape Industry?
Sustainability has emerged as a critical consideration for B2B buyers in the perineal fenestrated drape market. The environmental impact of medical waste, particularly from disposable products, is prompting healthcare organizations to seek greener alternatives. This shift is leading to an increased demand for drapes made from sustainable materials, such as nonwoven fabrics that are biodegradable or recyclable.
Ethical sourcing is equally important, as buyers are increasingly concerned about the labor practices and environmental policies of their suppliers. Certifications such as ISO 14001 for environmental management and OEKO-TEX for textile safety are becoming essential for manufacturers to demonstrate their commitment to sustainability. Buyers from regions like Europe and the Middle East, where consumer awareness of environmental issues is high, are particularly inclined to partner with suppliers who prioritize ethical practices.
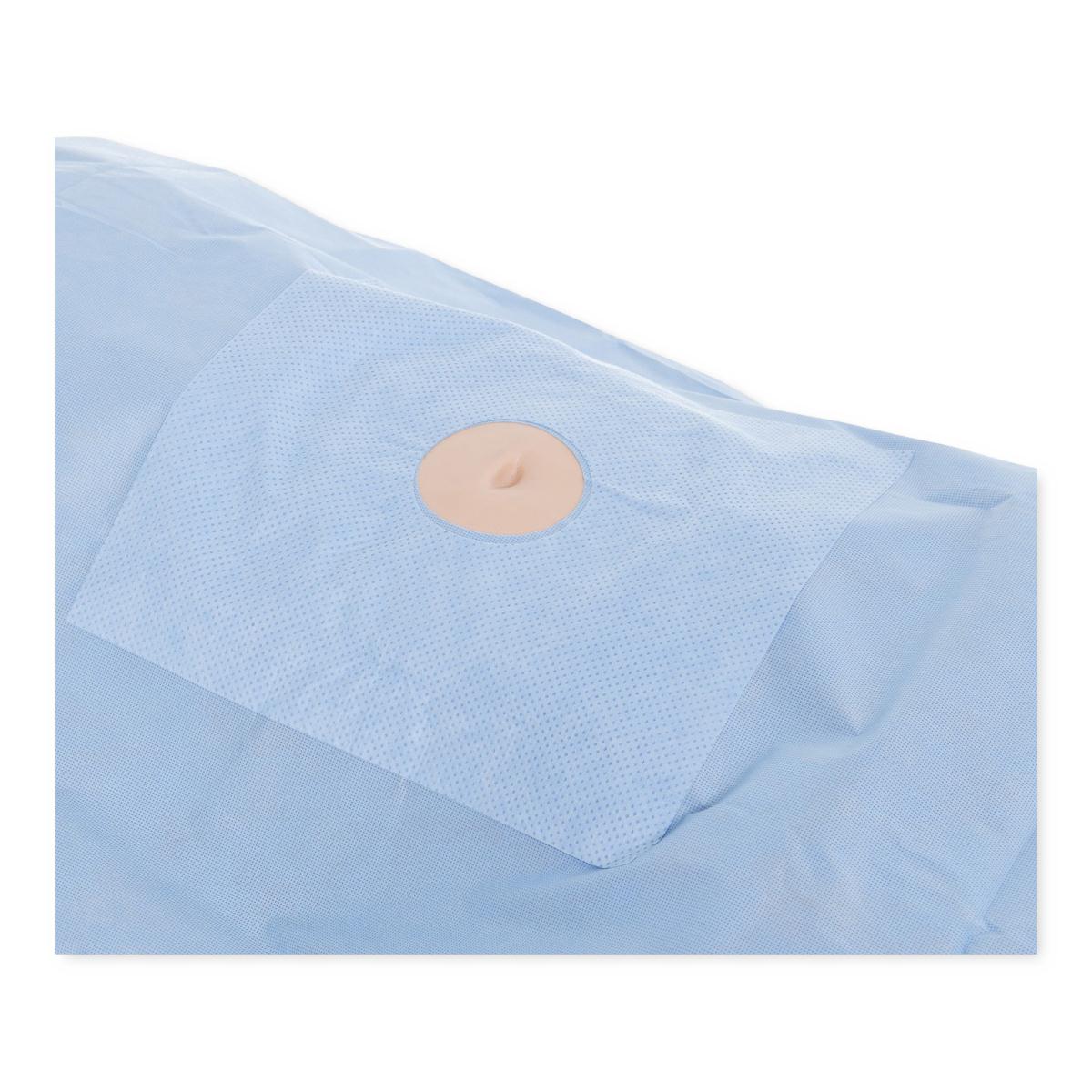
Illustrative image related to perineal fenestrated drape
The move towards sustainability is not just a regulatory necessity but also a market differentiator. Suppliers who can demonstrate environmentally friendly practices and materials are likely to gain a competitive edge. Therefore, international buyers should prioritize partnerships with manufacturers that align with their sustainability goals, ensuring a responsible and eco-conscious supply chain.
What Is the Historical Context of Perineal Fenestrated Drapes in the Medical Field?
The evolution of perineal fenestrated drapes can be traced back to the increasing need for specialized surgical products designed to enhance patient safety and procedural efficiency. Initially, surgical drapes were primarily made from basic cotton materials, which lacked the necessary properties to effectively manage fluid and reduce infection risk.
With advancements in materials science, the introduction of nonwoven fabrics revolutionized the industry, offering better barrier protection and sterility. Over the decades, the design of perineal fenestrated drapes has become more sophisticated, incorporating features such as reinforced edges, adhesive strips, and specific fenestration sizes tailored to various surgical procedures. This evolution reflects broader trends in the healthcare sector towards safety, efficiency, and innovation, making it essential for B2B buyers to stay informed about historical developments that influence current product offerings.
Frequently Asked Questions (FAQs) for B2B Buyers of perineal fenestrated drape
-
How do I ensure the quality of perineal fenestrated drapes from suppliers?
To ensure quality, request samples from potential suppliers before placing bulk orders. Look for certifications such as ISO 13485, which indicates compliance with international quality management standards for medical devices. Additionally, inquire about their manufacturing processes, material specifications, and quality control measures. Establishing a clear communication channel for quality assurance and conducting regular audits can further help maintain standards. -
What is the best material for perineal fenestrated drapes?
The best material for perineal fenestrated drapes is typically a high-quality non-woven fabric that offers barrier protection, is lightweight, and minimizes linting. Look for options that are sterile, latex-free, and resistant to liquids and tearing. Additionally, some products may feature reinforced areas to enhance durability during surgical procedures. Always consider the specific needs of your healthcare setting when selecting materials. -
What customization options are available for perineal fenestrated drapes?
Many manufacturers offer customization options for perineal fenestrated drapes, such as varying sizes, fenestration shapes, and additional features like adhesive strips or integrated pouches for instruments. It’s essential to communicate your specific requirements to suppliers during the procurement process. Customization can help optimize the drape’s functionality for particular surgical procedures, enhancing efficiency and patient safety. -
What are the typical minimum order quantities (MOQs) for perineal fenestrated drapes?
Minimum order quantities can vary significantly among suppliers, generally ranging from 50 to 500 units. When negotiating with suppliers, clarify their MOQ policies to ensure they align with your purchasing needs. Some suppliers may offer flexibility on MOQs for first-time buyers or during promotional periods, which can be advantageous for smaller healthcare facilities or new markets. -
What payment terms should I expect when sourcing from international suppliers?
Payment terms can vary widely based on the supplier’s policies and your negotiation. Common terms include 30% upfront payment with the balance due upon delivery, or net 30 to net 60 days after invoicing. Always confirm payment methods accepted (e.g., bank transfer, credit card, letter of credit) and consider discussing trade financing options to facilitate smoother transactions, especially for larger orders. -
How can I vet suppliers for perineal fenestrated drapes?
Vetting suppliers involves several steps: researching their business history, reading customer reviews, and checking for industry certifications. Establish direct communication to discuss your needs and assess their responsiveness. Request references from other clients in your region to gauge reliability. Additionally, consider visiting their manufacturing facility if possible, or utilizing third-party audits to ensure compliance with necessary standards. -
What logistics considerations should I keep in mind when importing perineal fenestrated drapes?
Logistics considerations include understanding import regulations in your country, shipping costs, and delivery timelines. Work closely with your supplier to confirm packaging standards and labeling requirements. It’s also advisable to partner with a reliable freight forwarder experienced in medical products to streamline customs clearance and ensure timely delivery. Additionally, consider inventory management strategies to maintain stock levels post-import. -
What are the key factors affecting the pricing of perineal fenestrated drapes?
Pricing for perineal fenestrated drapes is influenced by several factors, including material quality, manufacturing processes, order volume, and customization options. Additionally, geographical location, shipping costs, and import duties can affect final pricing. It’s advisable to compare quotes from multiple suppliers while considering the total cost of ownership, which includes quality, reliability, and service, not just the initial purchase price.
Top 6 Perineal Fenestrated Drape Manufacturers & Suppliers List
1. Medline – Sterile LAVH Surgical Drape
2. Grayline Medical – Welmed Drape Underleg Perineal Surgical CV Fenestrated
Domain: graylinemedical.com
Registered: 2019 (6 years)
Introduction: {“Product Name”: “Welmed Drape Underleg Perineal Surgical CV Fenestrated”, “Dimensions”: “84×8 inches”, “Quantity per Case”: “25”, “Price”: “$239.99”, “SKU”: “1222-5025”, “Type”: “Disposable”, “Fenestration”: “Fenestrated”, “Reinforcement”: “3×48 inches”, “Sterility”: “Sterile”, “Latex Content”: “Not Made With Natural Rubber Latex”, “Style”: “Bilateral”, “Processing Time”: “2-5 Business Days”, “Sh…
3. Halyard – EENT Fenestrated Drapes
Domain: products.halyardhealth.com
Registered: 2013 (12 years)
Introduction: HALYARD* EENT Fenestrated Drapes SKU P110407 Features: Maximum rating for flame resistance, Low-lint generation to reduce the risk of airborne bacterial transmission, Eye drapes designed with a unique U-shaped fluid-containment pouch that helps reduce exposure during phacoemulsification and other fluid-intensive procedures, may be used for the left or right eye, Product offerings include full or f…
4. Winner Medical – Laparotomy Surgical Drape
Domain: winnermedical.com
Registered: 1998 (27 years)
Introduction: {“description”: “Disposable surgical drape designed for laparotomy operations to prevent transfer of infections from surrounding areas to the wound.”, “benefits”: [“Meets needs for multiple drapes with abdominal and perineal apertures”, “Fenestrated incision film prevents transfer of infections”, “2-chamber instrument pouches for convenience within sterile field”, “Made from SMS or laminated fabri…
5. Dukal – Fenestrated Surgical Drapes
Domain: usamedicalsurgical.com
Registered: 2014 (11 years)
Introduction: {“Product Name”: “Dukal Fenestrated Surgical Drapes”, “Price”: “$24.11”, “Description”: “Fenestrated surgical drape providing access to the operative site while protecting the surrounding area. Economical surgical drapes for many minor surgical procedures.”, “Material”: “Tissue-poly-tissue with a polyethylene middle layer to eliminate risk of bacterial migration and moisture strike through.”, “Usa…
6. Welmed – Fenestrated Drape
Domain: welmed.us
Registered: 2006 (19 years)
Introduction: Fenestrated Drape Overall Dimensions: 22″ x 26″ 5” Adhesive Pad with 2” x 3” Oval Fenestration Item Number: 1220-176 Case Quantity: 400 Each/Case Latex Free ✓
Fenestrated Drape Overall Dimensions: 22″ x 26″ 5” Adhesive Pad with 2” x 3” Oval Fenestration Item Number: 1222-176 Case Quantity: 240 Each/Case Latex Free ✓
Fenestrated Drape Overall Dimensions: 30″ x 30″ 4″ x 6″ Fenestration with Surround…
Strategic Sourcing Conclusion and Outlook for perineal fenestrated drape
In the rapidly evolving medical landscape, strategic sourcing of perineal fenestrated drapes is paramount for healthcare providers aiming to enhance operational efficiency and patient safety. By investing in high-quality, disposable drapes, international buyers can mitigate infection risks and streamline surgical procedures. The variety of options available—from those with enhanced flame resistance to those featuring absorbent reinforcements—allows for tailored solutions that meet diverse procedural needs.
Furthermore, understanding regional supplier capabilities and compliance with local regulations can lead to significant cost savings and improved supply chain resilience. As global demand for surgical drapes continues to rise, particularly in markets across Africa, South America, the Middle East, and Europe, proactive engagement with reputable manufacturers will be crucial.
Looking ahead, buyers are encouraged to explore partnerships that prioritize innovation and sustainability in their product offerings. By doing so, they not only enhance their procurement strategies but also contribute to the overall advancement of healthcare standards. Now is the time to act—connect with trusted suppliers, evaluate your sourcing strategies, and ensure your organization is equipped for future challenges in surgical care.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.