How to Source Size Reduction Equipment For Pharmaceutical Industry Effectively: A 2025…
Introduction: Navigating the Global Market for size reduction equipment for pharmaceutical industry
In the ever-evolving pharmaceutical industry, sourcing the right size reduction equipment is crucial for ensuring product quality and operational efficiency. With the increasing demand for high-quality pharmaceuticals, international B2B buyers face the challenge of navigating a complex landscape filled with diverse equipment types and suppliers. This guide delves into the various categories of size reduction equipment, including crushers, grinders, and pulverizers, highlighting their specific applications and benefits tailored for pharmaceutical manufacturing.
By providing insights into supplier vetting processes, cost considerations, and the latest technological advancements, this comprehensive resource empowers buyers from regions such as Africa, South America, the Middle East, and Europe—including key markets like Vietnam and Saudi Arabia—to make informed purchasing decisions. Understanding the nuances of size reduction equipment not only enhances production efficiency but also aligns with regulatory standards and quality assurance protocols essential in the pharmaceutical sector.
As you explore this guide, you will gain actionable insights that facilitate the selection of the most suitable equipment, ensuring that your organization remains competitive in a global market characterized by rapid innovation and stringent quality demands. Whether you are a seasoned buyer or new to the industry, this resource will serve as an invaluable tool in your sourcing journey.
Understanding size reduction equipment for pharmaceutical industry Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Crushers | Heavy-duty machines for breaking large materials | Initial size reduction in bulk solids | Pros: High throughput; suitable for hard materials. Cons: Limited to coarse sizes; may require additional processing. |
| Grinders | Intermediate and fine grinding capabilities | Pharmaceutical powder production | Pros: Versatile for different particle sizes; efficient. Cons: May generate heat; requires careful monitoring of particle size. |
| Pulverizers | Produces very fine powders through high-energy impacts | Creation of fine pharmaceutical powders | Pros: Excellent for achieving micron and sub-micron sizes; precise control. Cons: Higher operational costs; potential wear on components. |
| Cutting Mills | Utilizes mechanical movement to cut materials | Processing soft to medium-hard solids | Pros: Effective for fibrous materials; low heat generation. Cons: Less effective for hard materials; may require more frequent maintenance. |
| High Shear Mixers | Combines mixing, emulsifying, and homogenizing | Formulation of emulsions and suspensions | Pros: Ensures uniformity; adaptable to various materials. Cons: Complexity in operation; requires skilled personnel. |
What Are Crushers and Their Suitability in Pharmaceuticals?
Crushers are robust machines designed to break down large solid materials into smaller, more manageable sizes. They are often the first step in the size reduction process, making them essential for processing bulk solids in the pharmaceutical industry. When selecting a crusher, buyers should consider the material’s hardness and the desired output size, as these factors will determine the machine’s efficiency and effectiveness.
How Do Grinders Enhance Pharmaceutical Powder Production?
Grinders are specialized for intermediate and fine grinding, transforming materials into powders suitable for pharmaceutical applications. They offer versatility in handling various materials and can achieve a range of particle sizes. Buyers must evaluate the grinder’s capacity, heat generation during operation, and the need for precise particle size control, as these factors influence product quality and production efficiency.
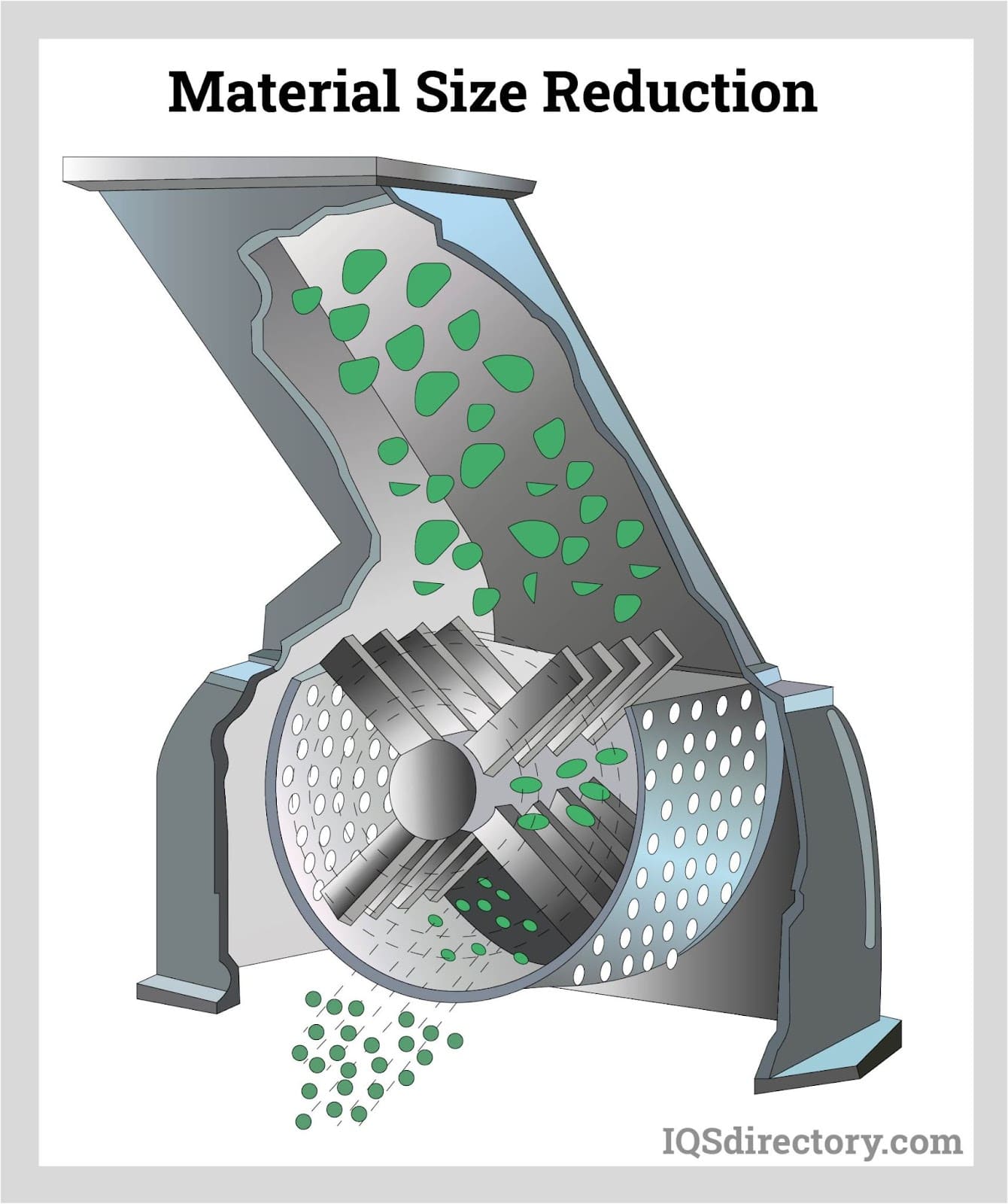
Illustrative image related to size reduction equipment for pharmaceutical industry
What Are the Advantages of Using Pulverizers for Fine Powder Creation?
Pulverizers are designed to produce extremely fine powders, which are critical in the pharmaceutical industry for creating high-quality formulations. They operate using high-energy impacts, allowing for precise control over particle size. When considering a pulverizer, B2B buyers should assess the operational costs, the material’s abrasiveness, and the potential wear on components, ensuring the selected equipment aligns with their production goals.
How Do Cutting Mills Function in Material Processing?
Cutting mills are effective for processing soft to medium-hard materials, utilizing mechanical movement to slice through materials rather than grinding them down. This makes them particularly useful for fibrous substances commonly found in pharmaceutical applications. Buyers should consider the material properties and the mill’s maintenance requirements, as these factors affect performance and longevity.
Why Are High Shear Mixers Important for Emulsions and Suspensions?
High shear mixers are crucial for creating emulsions and suspensions in the pharmaceutical industry, combining mixing, emulsifying, and homogenizing capabilities in one machine. Their ability to ensure uniformity in mixtures makes them invaluable for product consistency. When evaluating high shear mixers, B2B buyers should focus on the complexity of operation and the level of expertise required for effective use, as this can impact overall production efficiency.
Key Industrial Applications of size reduction equipment for pharmaceutical industry
| Industry/Sector | Specific Application of size reduction equipment for pharmaceutical industry | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Pharmaceutical Manufacturing | Granulation and powder preparation | Enhances bioavailability of drugs, ensuring effective dosing | Material compatibility, particle size requirements, and regulatory compliance |
| Nutraceuticals | Formulation of dietary supplements | Improves mixing efficiency and product consistency | Equipment versatility, capacity, and ease of cleaning |
| Biopharmaceuticals | Cell disruption for extraction of active ingredients | Maximizes yield of valuable compounds | Technology type, scalability, and energy consumption |
| Research & Development | Material characterization and sample preparation | Facilitates accurate analysis and testing | Precision of size reduction, reproducibility, and sample integrity |
| Quality Control | Homogenization for uniformity in formulations | Ensures product reliability and compliance with standards | Equipment reliability, maintenance support, and technical assistance |
How is Size Reduction Equipment Used in Pharmaceutical Manufacturing?
In pharmaceutical manufacturing, size reduction equipment plays a crucial role in granulation and powder preparation. This process enhances the bioavailability of active pharmaceutical ingredients (APIs), ensuring that medications are effective and deliver the correct dosage. Buyers from regions like Africa and South America must consider the compatibility of the equipment with various materials, the required particle size for optimal drug formulation, and adherence to stringent regulatory standards governing pharmaceutical production.
What Role Does Size Reduction Equipment Play in Nutraceuticals?
In the nutraceutical sector, size reduction equipment is essential for the formulation of dietary supplements. It facilitates improved mixing efficiency and consistency in product formulation, which is vital for maintaining quality across different batches. International buyers, particularly from the Middle East and Europe, should evaluate equipment versatility, production capacity, and ease of cleaning to ensure compliance with health regulations and to meet market demands effectively.
How is Size Reduction Equipment Utilized in Biopharmaceuticals?
For biopharmaceuticals, size reduction equipment is instrumental in cell disruption processes, allowing the extraction of valuable active ingredients from biological materials. This maximizes the yield of compounds necessary for drug formulation. Buyers must assess the type of technology employed, scalability for production needs, and energy consumption to ensure cost-effectiveness and sustainability in operations.
Why is Size Reduction Important in Research & Development?
In research and development, size reduction equipment is vital for material characterization and sample preparation. It ensures accurate analysis and testing by providing uniformly sized samples that enhance reproducibility. Buyers, particularly in Europe, should focus on the precision of the size reduction process, ensuring that it meets specific research requirements while maintaining sample integrity for reliable results.
How Does Size Reduction Equipment Contribute to Quality Control?
Size reduction equipment is also crucial in quality control processes, particularly for homogenization, which ensures uniformity in formulations. This contributes to product reliability and compliance with industry standards. Buyers should prioritize equipment reliability, maintenance support, and the availability of technical assistance to ensure continuous operation and adherence to quality benchmarks in their production lines.
3 Common User Pain Points for ‘size reduction equipment for pharmaceutical industry’ & Their Solutions
Scenario 1: Ensuring Consistent Particle Size for Drug Formulation
The Problem: A pharmaceutical manufacturer struggles with achieving consistent particle sizes during the milling process, leading to variations in drug efficacy and patient outcomes. The inconsistency not only affects the quality of the end product but also complicates regulatory compliance, as the pharmaceutical industry is heavily scrutinized for product uniformity. The buyer faces the daunting task of selecting the right equipment that can provide precision and reliability in size reduction while also adhering to stringent industry standards.
The Solution: To address this issue, buyers should focus on investing in high-quality milling equipment specifically designed for pharmaceutical applications. Utilizing a combination of particle size analysis tools and advanced milling technologies, such as high shear mixers or ball mills, can ensure that the desired particle size is consistently achieved. It is crucial to conduct a thorough material analysis before purchasing equipment to understand the properties of the substances being processed. This includes assessing hardness, moisture content, and other characteristics that influence the milling process. Furthermore, investing in equipment with adjustable settings allows for fine-tuning based on specific batch requirements, thus maintaining consistent quality across production runs.
Scenario 2: Managing High Production Costs Due to Inefficiencies
The Problem: A mid-sized pharmaceutical company is facing escalating production costs linked to outdated size reduction equipment. The inefficiencies stem from prolonged processing times and increased energy consumption, which ultimately cut into profit margins. The buyer is tasked with justifying the capital expenditure on new equipment while demonstrating the potential return on investment (ROI) to stakeholders.
The Solution: Buyers should consider upgrading to energy-efficient size reduction equipment that not only reduces energy consumption but also enhances throughput. Conducting a cost-benefit analysis can help in determining the potential savings from reduced operational costs. Additionally, exploring modular equipment options allows for gradual investment, where the company can start with a single unit that can be scaled up as production demands increase. Engaging with manufacturers who offer trial runs or equipment demonstrations can also provide insight into the efficiency of the equipment before making a final decision. This strategic approach allows buyers to make informed choices that align with both budget constraints and production goals.
Scenario 3: Navigating Regulatory Compliance Challenges
The Problem: A pharmaceutical organization is grappling with compliance issues related to the size reduction process, which is critical for meeting Good Manufacturing Practices (GMP). The buyer is concerned that their current equipment may not meet the latest regulatory standards, which could lead to costly fines or product recalls. This creates a pressing need for a reliable solution that ensures compliance without disrupting ongoing production.
The Solution: To mitigate compliance risks, buyers should prioritize sourcing size reduction equipment that is designed with regulatory standards in mind. This includes selecting machines that are easy to clean, have minimal cross-contamination risks, and come equipped with documentation for validation purposes. Collaborating with suppliers who are knowledgeable about the pharmaceutical industry and can provide equipment that meets GMP requirements is essential. Additionally, implementing a robust validation process for new equipment can ensure that it operates within the necessary specifications. Regular training for staff on compliance protocols and the correct operation of size reduction equipment can further reinforce adherence to industry standards, ultimately safeguarding the company’s reputation and financial stability.
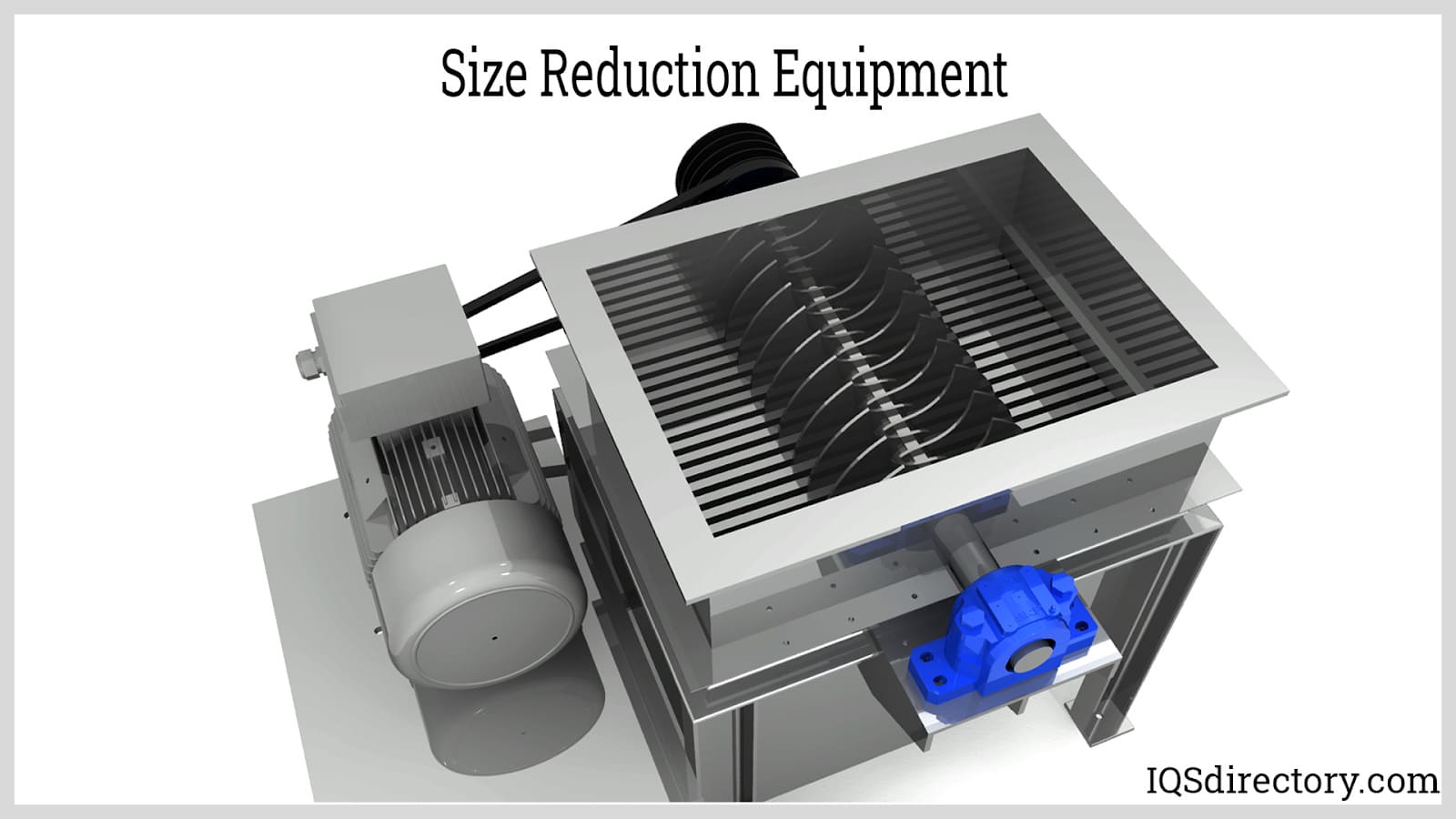
Illustrative image related to size reduction equipment for pharmaceutical industry
Strategic Material Selection Guide for size reduction equipment for pharmaceutical industry
What Are the Key Properties of Stainless Steel in Size Reduction Equipment?
Stainless steel is a widely used material in the pharmaceutical industry due to its excellent corrosion resistance, high strength, and ability to withstand high temperatures and pressures. Its non-reactive nature makes it suitable for processing active pharmaceutical ingredients (APIs) and excipients without contamination. The most common grades used include 304 and 316 stainless steel, with 316 offering superior resistance to chlorides and other corrosive agents.
Pros & Cons:
Stainless steel is durable and easy to clean, which is critical for maintaining hygiene in pharmaceutical applications. However, its higher cost compared to other materials can be a drawback, particularly for smaller manufacturers. The manufacturing complexity can also increase due to the need for specialized welding and finishing processes.
Impact on Application:
In size reduction equipment, stainless steel’s compatibility with various media makes it ideal for grinding and milling processes. Its smooth surface minimizes the risk of particle adhesion, ensuring consistent product quality.
Considerations for International Buyers:
Buyers should ensure compliance with international standards such as ASTM and ISO for material selection. In regions like Africa and South America, where cost sensitivity is higher, the initial investment in stainless steel may be justified by its long-term durability and lower maintenance costs.
How Does Plastic Compare as a Material for Size Reduction Equipment?
Plastics, particularly high-performance polymers like polyether ether ketone (PEEK) and polyvinylidene fluoride (PVDF), are increasingly used in size reduction equipment. These materials offer good chemical resistance and can operate effectively at moderate temperatures.
Pros & Cons:
The primary advantage of plastics is their lightweight nature and lower cost compared to metals. They are also less prone to corrosion. However, they may not withstand the same high temperatures and pressures as metals, which can limit their applications. Additionally, the durability of plastics can be a concern, especially in high-wear environments.
Impact on Application:
Plastics are particularly suitable for processing sensitive materials that require a non-reactive environment. Their flexibility in design allows for innovative equipment configurations.
Considerations for International Buyers:
Buyers must consider local regulations regarding the use of plastics in food and pharmaceutical applications. Compliance with standards such as FDA and EU regulations is essential, particularly in Europe and the Middle East.
What Role Does Ceramic Material Play in Size Reduction Equipment?
Ceramic materials, especially those engineered for wear resistance, are used in size reduction equipment due to their hardness and durability. They are often found in high-performance grinding applications where metal contamination must be avoided.
Pros & Cons:
Ceramics offer excellent wear resistance and can handle high temperatures, making them ideal for abrasive materials. However, they can be brittle and susceptible to cracking under impact, which may limit their use in certain applications. The cost of ceramic components can also be higher due to manufacturing complexities.
Impact on Application:
Ceramics are particularly effective in applications requiring high purity, such as in the production of pharmaceutical powders. Their inert nature ensures that no unwanted reactions occur during processing.
Considerations for International Buyers:
Buyers should be aware of the specific grades of ceramics required for their applications and ensure compliance with relevant international standards. In regions like Africa and South America, where budget constraints exist, the investment in ceramics should be weighed against their performance benefits.
Why Is Carbon Steel Considered for Size Reduction Equipment?
Carbon steel is often used in size reduction equipment for its strength and cost-effectiveness. It is suitable for applications where corrosion resistance is not a primary concern, and it can be treated with coatings to enhance its durability.
Pros & Cons:
The main advantage of carbon steel is its low cost and high strength, making it an attractive option for many manufacturers. However, its susceptibility to rust and corrosion can be a significant drawback, especially in humid environments or when processing corrosive materials.
Impact on Application:
Carbon steel is commonly used in crushers and shredders where the material being processed is less sensitive to contamination. Its robust nature allows it to handle large volumes of material effectively.
Considerations for International Buyers:
International buyers should consider the environmental conditions of their operations. In regions with high humidity, additional protective coatings may be necessary to prolong the lifespan of carbon steel equipment.
| Material | Typical Use Case for Size Reduction Equipment for Pharmaceutical Industry | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Stainless Steel | Grinding and milling of APIs and excipients | Excellent corrosion resistance | Higher cost and manufacturing complexity | High |
| Plastic | Processing sensitive materials in a non-reactive environment | Lightweight and lower cost | Limited temperature and pressure tolerance | Medium |
| Ceramic | High-performance grinding applications for high purity powders | Exceptional wear resistance | Brittle and higher manufacturing costs | High |
| Carbon Steel | Crushers and shredders for less sensitive materials | Low cost and high strength | Susceptible to rust and corrosion | Low |
In-depth Look: Manufacturing Processes and Quality Assurance for size reduction equipment for pharmaceutical industry
What are the Key Stages in the Manufacturing Process of Size Reduction Equipment for the Pharmaceutical Industry?
The manufacturing process of size reduction equipment specifically designed for the pharmaceutical sector involves several critical stages, ensuring that the final product meets stringent industry standards for safety, efficiency, and reliability.
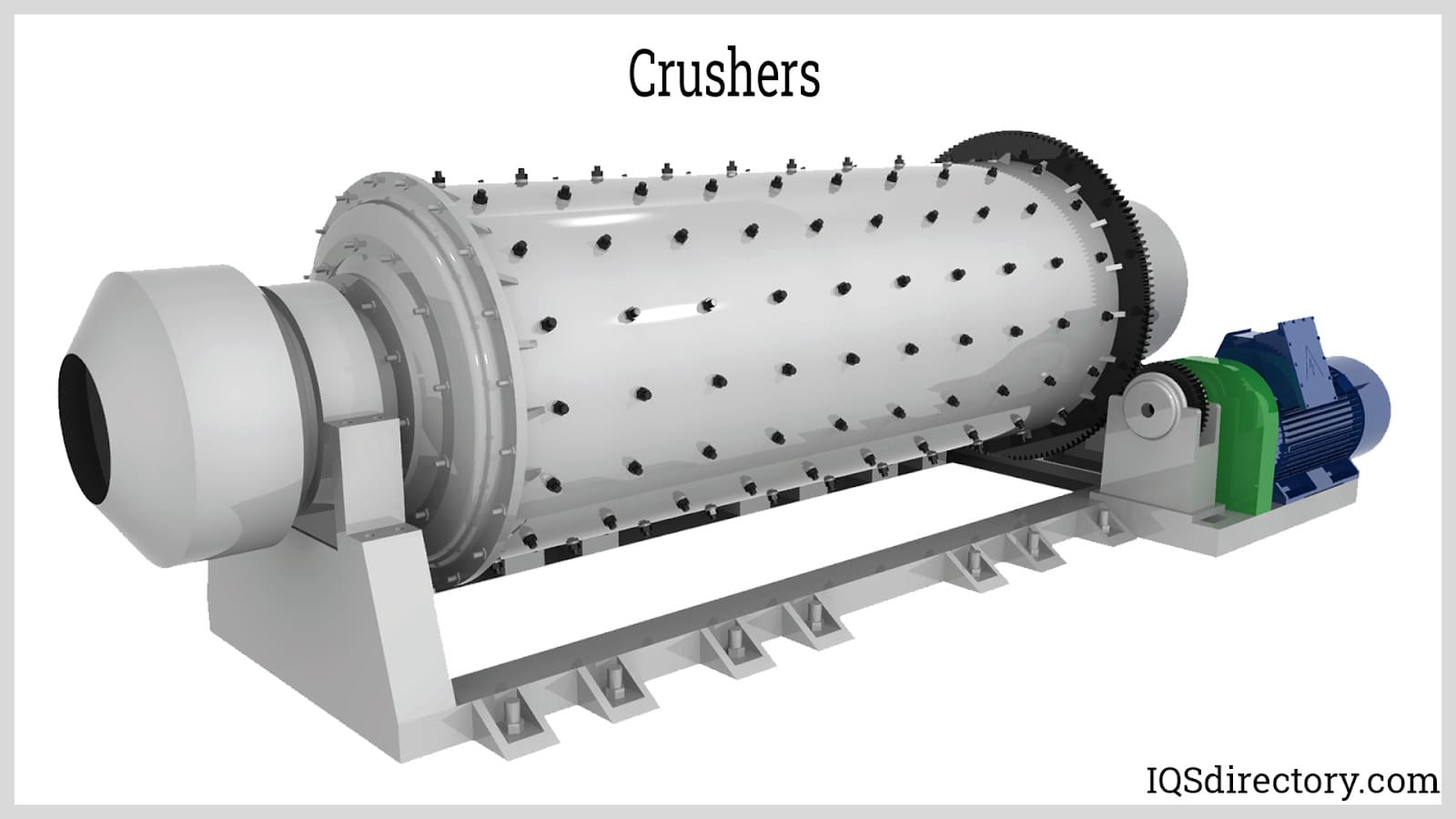
Illustrative image related to size reduction equipment for pharmaceutical industry
Material Preparation: How is Raw Material Selected and Processed?
The first stage is material preparation, which involves selecting high-quality raw materials that meet the specific requirements of the pharmaceutical industry. Common materials include stainless steel for its resistance to corrosion and ease of cleaning, as well as specialized alloys for components subject to wear.
Once selected, materials undergo a series of pre-processing steps, such as cutting, grinding, or screening, to ensure they meet dimensional and compositional specifications. This phase often includes quality checks to verify that the materials conform to relevant standards, such as ASTM or ISO specifications, which are crucial for ensuring that the end equipment will perform reliably in a pharmaceutical environment.
What Techniques are Used in the Forming Stage of Size Reduction Equipment?
Following material preparation, the forming stage involves shaping the materials into specific components necessary for size reduction equipment. Techniques such as machining, welding, and casting are commonly employed.
Machining processes may include CNC milling and turning, which offer precision and flexibility for creating complex geometries. For components requiring high strength, welding techniques ensure robust joints that can withstand operational stresses. Casting may be utilized for larger components, allowing for intricate designs that would be difficult to achieve with other methods.
How is Assembly Conducted for Size Reduction Equipment?
Once the individual components are formed, the assembly process begins. This stage is critical as it brings together all parts to create the final equipment. Assembly may involve mechanical fastening, adhesive bonding, or welding, depending on the design and material properties.
During assembly, technicians must adhere to strict protocols to ensure that all components fit together correctly and function as intended. This includes aligning parts precisely, checking for mechanical tolerances, and ensuring that all moving parts operate smoothly. This phase is often accompanied by real-time quality checks to identify any deviations from specifications before the equipment moves to the finishing stage.
What Finishing Techniques Are Applied to Size Reduction Equipment?
The finishing stage enhances the equipment’s aesthetic and functional qualities. Techniques such as surface treatment, polishing, and coating are employed to improve corrosion resistance and reduce friction during operation.
Surface treatments, like passivation, are crucial in pharmaceutical applications to ensure that the equipment does not contaminate sensitive materials. Furthermore, finishing processes may also include rigorous cleaning and sterilization to prepare the equipment for its intended use in the pharmaceutical environment.

Illustrative image related to size reduction equipment for pharmaceutical industry
What Quality Assurance Standards Should B2B Buyers Consider for Size Reduction Equipment?
Quality assurance is paramount in the manufacturing of size reduction equipment for the pharmaceutical industry. International standards, such as ISO 9001, provide a framework for ensuring that manufacturers consistently meet customer and regulatory requirements.
Which International Standards are Relevant to Size Reduction Equipment?
For the pharmaceutical industry, compliance with industry-specific standards such as CE marking (Conformité Européenne) and API (Active Pharmaceutical Ingredients) guidelines is essential. CE marking indicates that the equipment meets European health, safety, and environmental protection standards, while API guidelines ensure that the equipment is suitable for processing active pharmaceutical ingredients.
Additionally, adherence to Good Manufacturing Practices (GMP) is critical. GMP regulations outline the necessary processes and documentation required to ensure that products are consistently produced and controlled according to quality standards.
What are the Key Quality Control Checkpoints in Manufacturing?
Quality control (QC) checkpoints play a vital role in maintaining the integrity of the manufacturing process. Key checkpoints typically include:
-
Incoming Quality Control (IQC): This phase involves inspecting raw materials upon arrival to ensure they meet specified standards before they enter the production process.
-
In-Process Quality Control (IPQC): Throughout the manufacturing stages, periodic checks are conducted to monitor processes and detect any deviations early, ensuring that corrective actions can be taken.
-
Final Quality Control (FQC): After assembly, the final product undergoes a thorough inspection and testing phase. This includes functional testing, performance validation, and compliance checks against international and industry standards.
How Can B2B Buyers Verify Supplier Quality Control Processes?
B2B buyers should take proactive steps to verify the quality control processes of their suppliers to ensure that the size reduction equipment meets their specific requirements.
What Methods Can Be Employed for Supplier Audits?
-
Supplier Audits: Conducting on-site audits allows buyers to assess the manufacturing environment, processes, and quality control measures firsthand. This provides insights into the supplier’s adherence to ISO standards and other relevant certifications.
-
Quality Control Reports: Requesting documentation of quality control processes, including IQC, IPQC, and FQC results, can help verify compliance with industry standards and the reliability of the manufacturing process.
-
Third-Party Inspections: Engaging third-party inspection services can offer an unbiased evaluation of the supplier’s quality control practices. These services can assess compliance with international standards and provide detailed reports on manufacturing processes and outcomes.
What Unique Challenges Do International B2B Buyers Face Regarding Quality Control?
International buyers from regions such as Africa, South America, the Middle East, and Europe face unique challenges when it comes to quality control in the procurement of size reduction equipment.
How Can Cultural and Regulatory Differences Affect Quality Assurance?
Cultural differences and varying regulatory requirements can complicate the quality assurance process. Buyers must navigate different standards and expectations in the countries they operate within. For instance, understanding local regulations in Saudi Arabia or Vietnam regarding equipment safety and performance may require additional research and local expertise.
Moreover, language barriers can hinder effective communication regarding quality requirements, making it crucial for buyers to engage with suppliers who are familiar with international standards and practices.
Conclusion
The manufacturing processes and quality assurance measures for size reduction equipment tailored for the pharmaceutical industry are complex and must adhere to stringent standards. By understanding the key stages of manufacturing and the quality control checkpoints, B2B buyers can make informed decisions when sourcing equipment. Additionally, verifying supplier quality control processes through audits, reports, and third-party inspections can significantly mitigate risks associated with procurement, ensuring that the equipment meets the required standards for safety and efficiency in pharmaceutical applications.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘size reduction equipment for pharmaceutical industry’
This guide provides a structured approach for B2B buyers in the pharmaceutical industry looking to procure size reduction equipment. Proper selection is crucial, as it directly impacts product quality, efficiency, and compliance with industry standards. Follow these steps to ensure a successful procurement process.
Step 1: Define Your Technical Specifications
Clearly outline the technical requirements for the size reduction equipment you need. Consider the material properties, desired particle size, and production capacity.
– Material Characteristics: Assess hardness, moisture content, and abrasiveness to determine the most suitable equipment.
– Particle Size Requirements: Specify the required final fineness, as this will influence the type of equipment needed—crushers for larger sizes and mills for finer reductions.
Step 2: Research Available Equipment Types
Familiarize yourself with the various types of size reduction equipment available in the market.
– Crushers vs. Grinders: Understand the differences; crushers are ideal for initial size reduction, while grinders refine materials further.
– Specialized Equipment: Consider equipment like pulverizers and cutting mills that cater to specific needs within the pharmaceutical sector.
Step 3: Evaluate Potential Suppliers
Before committing to a supplier, conduct thorough evaluations.
– Company Profiles: Request detailed company information, including their history, expertise, and market presence.
– References and Case Studies: Seek testimonials from other pharmaceutical companies that have utilized their equipment, particularly in similar applications or regions.
Step 4: Verify Compliance with Industry Standards
Ensure that the equipment meets relevant industry regulations and standards.
– Quality Certifications: Look for suppliers with ISO certifications or other relevant quality assurances.
– Compliance with Regulations: Confirm that the equipment complies with pharmaceutical manufacturing regulations, such as GMP (Good Manufacturing Practices).
Step 5: Assess After-Sales Support and Maintenance Services
Evaluate the supplier’s after-sales support and maintenance offerings.
– Technical Support: Ensure that the supplier provides comprehensive technical assistance for installation and operation.
– Maintenance Services: Investigate whether they offer ongoing maintenance contracts to ensure long-term reliability and performance of the equipment.
Step 6: Request Demonstrations and Samples
Whenever possible, request product demonstrations or trial runs to assess the equipment’s performance.
– Performance Evaluation: Observe how the equipment handles your specific materials and meets your size reduction requirements.
– Sample Testing: If applicable, ask for sample runs to evaluate the quality of the output before making a significant investment.

Illustrative image related to size reduction equipment for pharmaceutical industry
Step 7: Negotiate Terms and Finalize Your Purchase
Once you have selected a supplier, engage in negotiations to finalize the purchase terms.
– Pricing and Payment Terms: Discuss pricing structures, potential discounts, and payment schedules.
– Delivery and Installation: Confirm delivery timelines and installation requirements to align with your production schedule.
By following this checklist, B2B buyers in the pharmaceutical industry can make informed decisions when sourcing size reduction equipment, ultimately leading to enhanced operational efficiency and product quality.
Comprehensive Cost and Pricing Analysis for size reduction equipment for pharmaceutical industry Sourcing
What Are the Key Cost Components for Sizing Reduction Equipment in the Pharmaceutical Industry?
When analyzing the costs associated with size reduction equipment for the pharmaceutical industry, various components must be considered. The primary cost components include materials, labor, manufacturing overhead, tooling, quality control (QC), logistics, and profit margins.
-
Materials: The choice of materials significantly impacts the overall cost. High-grade stainless steel and specialized alloys are often required for pharmaceutical applications to ensure compliance with hygiene and safety standards.
-
Labor: Labor costs encompass both skilled technicians for assembly and unskilled labor for basic operations. The complexity of the equipment often dictates the level of expertise required, influencing labor costs.
-
Manufacturing Overhead: This includes expenses related to utilities, rent, and equipment depreciation. Overhead costs can vary widely based on geographical location and the efficiency of manufacturing processes.
-
Tooling: Custom tooling may be necessary for specialized equipment designs, which can add to the initial cost but may improve efficiency and reduce long-term operational costs.
-
Quality Control (QC): Rigorous quality testing is essential in the pharmaceutical industry. QC processes ensure that the equipment meets stringent regulatory standards, which can add to the overall cost.
-
Logistics: Shipping and handling costs must be factored in, especially for international buyers. The complexity of the equipment and the distance to the destination can significantly influence logistics expenses.
-
Margin: Suppliers typically apply a profit margin on top of the total cost structure, which can vary based on market demand, competition, and the supplier’s position in the market.
How Do Price Influencers Affect Sizing Reduction Equipment Costs?
Several factors can influence the pricing of size reduction equipment, particularly for international B2B buyers.
-
Volume/MOQ: Larger order quantities often lead to reduced per-unit costs due to economies of scale. Buyers should negotiate minimum order quantities (MOQs) that align with their production needs.
-
Specifications and Customization: Custom equipment designed to meet specific processing requirements will typically incur higher costs. Buyers must evaluate whether standard equipment can meet their needs to avoid unnecessary expenses.
-
Materials and Quality Certifications: The choice of materials and the presence of quality certifications (like ISO or GMP) can also affect pricing. Equipment that meets higher standards may command a premium.
-
Supplier Factors: The reputation, location, and reliability of suppliers can influence pricing. Established suppliers with proven track records may charge more, but they can also offer better support and reliability.
-
Incoterms: Understanding Incoterms is critical for international transactions. Different terms can affect who bears the cost of shipping, insurance, and tariffs, ultimately influencing the total cost.
What Buyer Tips Can Help Navigate Costs and Pricing Nuances?
For international buyers, particularly from regions like Africa, South America, the Middle East, and Europe, understanding the nuances of pricing and cost management is crucial.

Illustrative image related to size reduction equipment for pharmaceutical industry
-
Negotiation: Always negotiate pricing, especially for larger orders. Suppliers may have room to adjust their margins based on volume commitments.
-
Cost-Efficiency: Evaluate the total cost of ownership (TCO), which includes not just the purchase price but also maintenance, operation, and potential downtime costs. This holistic view can lead to better long-term investment decisions.
-
Pricing Nuances: Be aware of fluctuations in currency exchange rates, which can affect pricing for international buyers. Locking in prices or using hedging strategies can mitigate risks.
-
Supplier Relationships: Building strong relationships with suppliers can lead to more favorable terms, faster turnaround times, and improved communication, which is invaluable in the complex pharmaceutical landscape.
Conclusion and Disclaimer
While the insights provided here offer a comprehensive overview of cost structures and pricing factors for size reduction equipment in the pharmaceutical industry, it is essential to consult directly with suppliers for specific pricing. Indicative prices can vary based on market conditions, customization requirements, and geographical factors.
Alternatives Analysis: Comparing size reduction equipment for pharmaceutical industry With Other Solutions
Introduction: What Are the Alternatives to Size Reduction Equipment in the Pharmaceutical Industry?
In the pharmaceutical industry, size reduction is a critical process that affects product quality, bioavailability, and manufacturing efficiency. While specialized size reduction equipment such as mills and pulverizers are widely used, several alternative solutions can also achieve similar objectives. Understanding these alternatives can help B2B buyers make informed decisions based on performance, cost, and operational needs.
Comparison Table
| Comparison Aspect | Size Reduction Equipment For Pharmaceutical Industry | High Shear Mixers | Cryogenic Grinding |
|---|---|---|---|
| Performance | High precision in achieving desired particle size | Excellent for emulsifying and homogenizing | Produces fine particles without heat |
| Cost | Generally high initial investment | Moderate, depending on complexity | High due to specialized equipment |
| Ease of Implementation | Requires specialized training and setup | Easier to integrate into existing processes | Complex setup and operational requirements |
| Maintenance | Regular maintenance required for optimal performance | Moderate, with some wear on parts | High maintenance due to cooling systems |
| Best Use Case | Ideal for controlled particle size in tablets | Best for liquid formulations and creams | Suitable for heat-sensitive materials |
Detailed Breakdown of Alternatives
High Shear Mixers: Pros and Cons
High shear mixers are designed to blend and emulsify materials by applying intense mechanical energy. They are particularly beneficial for producing homogeneous mixtures, making them ideal for liquid formulations in the pharmaceutical sector. Their ease of integration into existing processes is a significant advantage, as they can be used alongside other mixing equipment. However, while they excel in emulsifying, they may not achieve the same level of particle size reduction as dedicated size reduction equipment. Their moderate cost and maintenance needs make them a viable option for companies looking for versatility in their mixing processes.
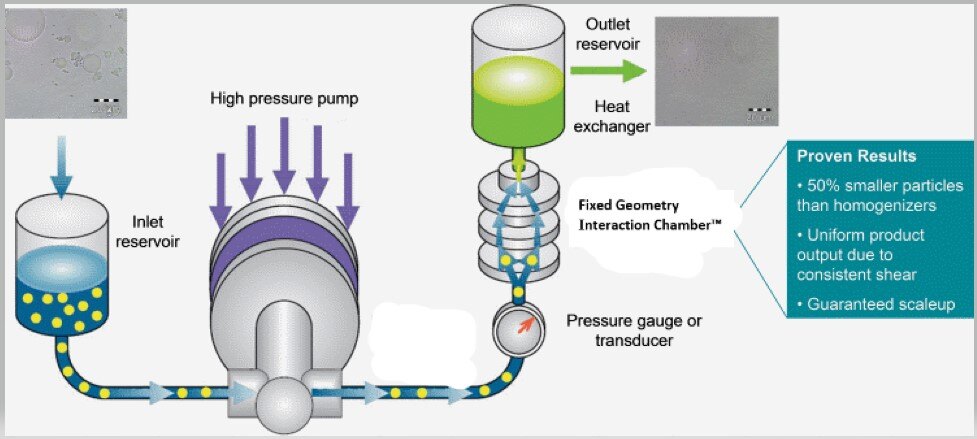
Illustrative image related to size reduction equipment for pharmaceutical industry
Cryogenic Grinding: Pros and Cons
Cryogenic grinding employs extremely low temperatures to embrittle materials before size reduction. This method is particularly advantageous for heat-sensitive substances, as it prevents degradation during the grinding process. The resulting fine particles often exhibit improved flow properties and better bioavailability. However, the initial investment in cryogenic systems can be substantial, and the complexity of operation requires specialized training. Additionally, the maintenance of cooling systems can add to operational costs, making it less accessible for smaller pharmaceutical manufacturers.
Conclusion: How to Choose the Right Solution for Your Pharmaceutical Needs
Selecting the appropriate size reduction method requires a careful analysis of your specific requirements. Consider factors such as the nature of the materials being processed, desired particle size, and operational capabilities. For instance, if your focus is on achieving precise particle sizes for tablets, size reduction equipment may be your best bet. Conversely, if you are dealing with liquid formulations, high shear mixers could offer greater efficiency. For heat-sensitive materials, cryogenic grinding may be the ideal solution despite its higher costs. Ultimately, understanding the unique needs of your production processes will guide you in making the best choice for your pharmaceutical manufacturing operations.
Essential Technical Properties and Trade Terminology for size reduction equipment for pharmaceutical industry
What Are the Key Technical Properties of Size Reduction Equipment for the Pharmaceutical Industry?
When selecting size reduction equipment for the pharmaceutical sector, understanding specific technical properties is crucial for ensuring operational efficiency and compliance with industry standards. Here are some essential specifications to consider:
-
Material Grade
The material grade of the equipment, such as stainless steel or specialized alloys, is vital for maintaining hygiene and preventing contamination. In the pharmaceutical industry, equipment must often adhere to stringent regulatory standards, making high-grade materials essential for product safety and compliance. -
Particle Size Distribution
The ability to achieve a specific particle size distribution is critical in pharmaceuticals, where the efficacy of active ingredients can be affected by particle size. Equipment should be capable of producing uniform particle sizes to ensure consistent dosing and enhanced bioavailability of drugs. -
Throughput Capacity
This specification refers to the volume of material that can be processed in a given time frame. For pharmaceutical manufacturers, selecting equipment with the right throughput capacity is essential for meeting production targets and maintaining efficiency in the supply chain. -
Tolerance Levels
Tolerance levels determine the acceptable deviation from specified dimensions during the size reduction process. Tight tolerances are often required in pharmaceutical applications to ensure that the final product meets regulatory standards and performs as intended. -
Power Consumption
Energy efficiency is increasingly important in the pharmaceutical industry due to rising operational costs. Equipment that utilizes power efficiently can reduce operational expenses and support sustainability initiatives. -
Ease of Cleaning and Maintenance
Given the stringent hygiene requirements in the pharmaceutical sector, equipment should be designed for easy disassembly and cleaning. This property not only aids compliance with health regulations but also minimizes downtime during maintenance.
What Are Common Trade Terms Used in Size Reduction Equipment Procurement?
Navigating the procurement process for size reduction equipment requires familiarity with industry-specific terminology. Here are several key terms that B2B buyers should understand:
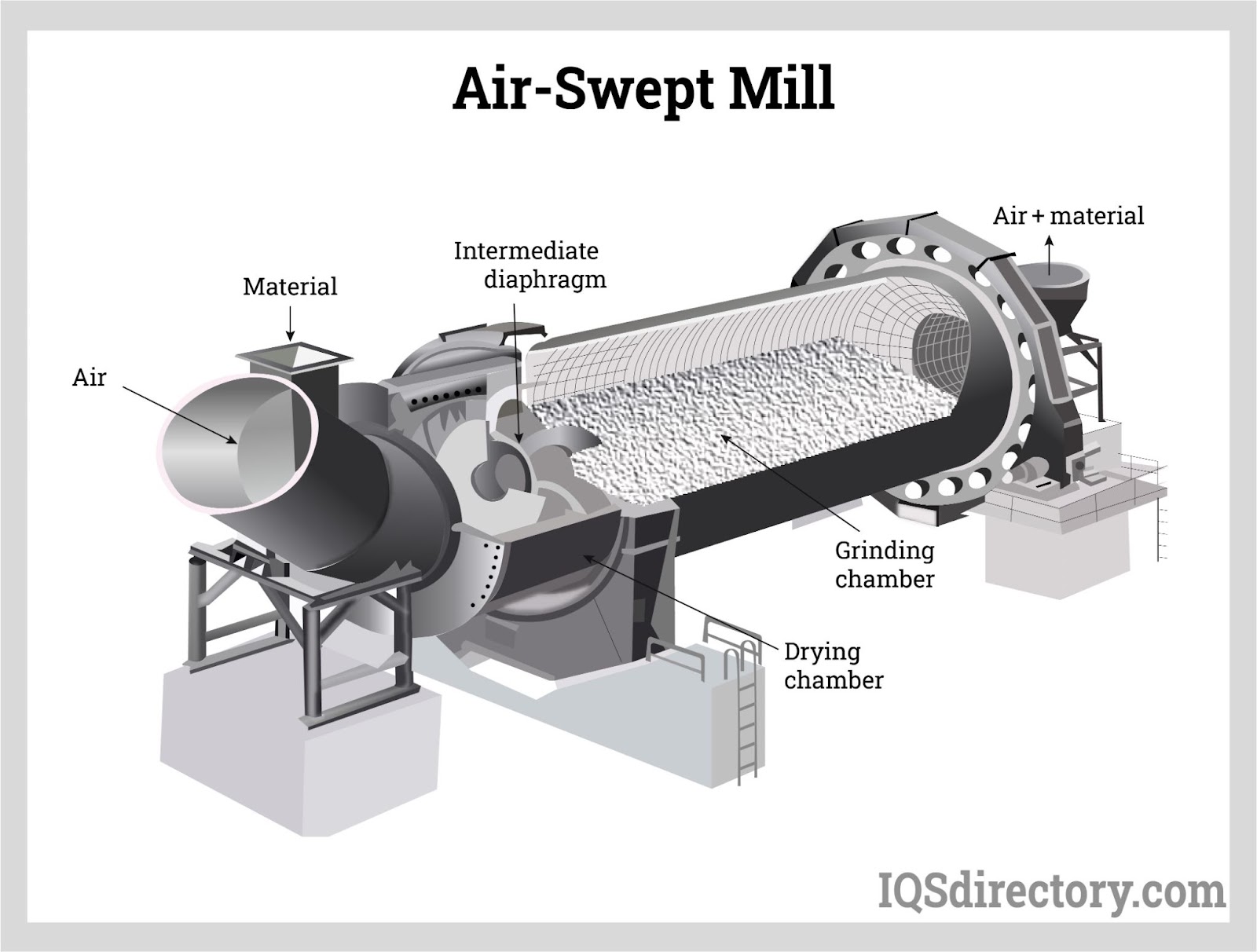
Illustrative image related to size reduction equipment for pharmaceutical industry
-
OEM (Original Equipment Manufacturer)
An OEM refers to a company that produces parts or equipment that may be marketed by another manufacturer. In the context of size reduction equipment, understanding whether you are dealing with an OEM can affect warranty terms, support, and parts availability. -
MOQ (Minimum Order Quantity)
This term indicates the smallest quantity of a product that a supplier is willing to sell. Knowing the MOQ is crucial for buyers to avoid overcommitting to large orders that may exceed their immediate needs or budget. -
RFQ (Request for Quotation)
An RFQ is a standard business process where buyers invite suppliers to submit price proposals for specific products or services. For size reduction equipment, issuing an RFQ helps buyers compare offers, ensuring they get the best value for their investment. -
Incoterms (International Commercial Terms)
These are international rules that define the responsibilities of buyers and sellers regarding the delivery of goods. Understanding Incoterms is essential for B2B transactions as they clarify shipping costs, risks, and insurance responsibilities. -
Lead Time
This term refers to the amount of time between placing an order and receiving the equipment. For the pharmaceutical industry, where timelines can be critical, understanding lead times helps in planning production schedules and inventory management. -
Certification Standards
These are regulatory benchmarks that size reduction equipment must meet to ensure safety and efficacy. Familiarity with relevant certification standards, such as cGMP (current Good Manufacturing Practices), is essential for compliance in the pharmaceutical sector.
By understanding these technical properties and trade terms, B2B buyers in the pharmaceutical industry can make informed decisions that enhance operational efficiency and product quality.
Navigating Market Dynamics and Sourcing Trends in the size reduction equipment for pharmaceutical industry Sector
What Are the Current Market Dynamics and Key Trends in Size Reduction Equipment for the Pharmaceutical Industry?
The global market for size reduction equipment in the pharmaceutical sector is experiencing significant transformation, driven by increasing demand for efficient manufacturing processes and stringent regulatory requirements. A key trend is the growing adoption of advanced technologies such as automation and artificial intelligence, which enhance operational efficiency and reduce human error. International buyers, particularly from emerging markets in Africa, South America, the Middle East, and Europe, are increasingly seeking equipment that integrates these technologies for improved production capabilities.
Another notable trend is the focus on customized solutions. Pharmaceutical companies are looking for size reduction equipment tailored to their specific production needs, whether it’s for particle size optimization or compliance with unique regulatory standards. This has led to greater collaboration between equipment manufacturers and pharmaceutical firms, fostering innovation and the development of specialized machinery. Additionally, the rise of contract manufacturing organizations (CMOs) is influencing sourcing strategies, as these entities often require versatile equipment capable of handling diverse product lines.
Sustainability is also becoming a significant consideration. Buyers are increasingly prioritizing suppliers that demonstrate commitment to environmentally friendly practices, leading to a surge in demand for energy-efficient and waste-reducing equipment. This shift is particularly prominent in regions like Europe and North America, where regulatory frameworks are stringent, but it is also gaining traction in developing markets.
How Is Sustainability Shaping the Sourcing of Size Reduction Equipment in the Pharmaceutical Sector?
Sustainability has emerged as a critical factor influencing the sourcing of size reduction equipment in the pharmaceutical industry. Environmental impact assessments are becoming standard practice, with buyers seeking equipment that minimizes energy consumption and reduces waste generation. This shift is not only about compliance but also about enhancing brand reputation and meeting consumer expectations for environmentally responsible practices.
Ethical sourcing is intertwined with sustainability, with many companies emphasizing the importance of transparent supply chains. Buyers are increasingly interested in suppliers who adhere to ethical labor practices and provide clear documentation regarding the origin of materials used in manufacturing size reduction equipment. Certifications such as ISO 14001 for environmental management and ISO 45001 for occupational health and safety are gaining importance, as they provide assurance of a supplier’s commitment to sustainable practices.
The trend towards ‘green’ materials is also significant. Manufacturers are investing in research to develop equipment that utilizes recyclable or biodegradable materials, thereby reducing the overall environmental footprint. For B2B buyers, this not only aligns with corporate social responsibility goals but also enhances the sustainability profile of their operations, making their products more appealing in an increasingly eco-conscious market.
What Is the Brief Evolution and History of Size Reduction Equipment in the Pharmaceutical Sector?
The evolution of size reduction equipment in the pharmaceutical industry has been marked by technological advancements and changing production methodologies. Initially, size reduction processes were rudimentary, relying on manual techniques for grinding and milling. However, as the pharmaceutical sector expanded and regulatory requirements tightened, there was a need for more sophisticated machinery capable of producing finer, more uniform particles.
The introduction of electric-powered mills and grinders in the mid-20th century revolutionized the industry, allowing for greater precision and efficiency. Over the years, innovations such as high shear mixers and advanced pulverizers have emerged, enabling manufacturers to meet the stringent quality standards required for pharmaceutical products. Today, the focus is on integrating automation and data analytics to enhance production processes, reflecting a broader trend towards Industry 4.0 within the sector.
The historical trajectory of size reduction equipment illustrates a shift from manual processes to high-tech solutions, underscoring the industry’s commitment to continuous improvement and adaptation to market demands. As the pharmaceutical landscape evolves, so too will the technologies and methodologies employed in size reduction, ensuring that companies remain competitive in an ever-changing global marketplace.
Frequently Asked Questions (FAQs) for B2B Buyers of size reduction equipment for pharmaceutical industry
-
How do I choose the right size reduction equipment for my pharmaceutical manufacturing process?
Selecting the appropriate size reduction equipment hinges on several factors, including the material properties (hardness, moisture content), desired particle size, and production volume. Begin by assessing the physical characteristics of the materials you’ll be processing. For example, harder materials may require crushers or pulverizers, while softer materials might be best suited for cutting mills. Additionally, consider operational efficiency, maintenance needs, and scalability for future growth. Collaborating with equipment suppliers can also provide tailored solutions that meet your specific requirements. -
What are the key benefits of using size reduction equipment in the pharmaceutical industry?
The primary benefits of size reduction equipment in pharmaceuticals include enhanced product uniformity, increased surface area for improved processing, and better extraction efficiency. Proper size reduction aids in achieving consistent particle sizes, which is crucial for formulation quality and bioavailability. Moreover, it streamlines downstream processes such as mixing and tableting, ultimately leading to higher product quality and reduced production costs. Leveraging advanced equipment can also facilitate compliance with stringent industry regulations regarding product consistency and quality. -
What are the international shipping considerations for purchasing size reduction equipment?
When sourcing size reduction equipment internationally, it’s essential to consider shipping logistics, including import/export regulations, customs duties, and transportation costs. Ensure that your supplier is experienced in handling international shipments and can provide necessary documentation, such as certificates of origin and compliance. Additionally, factor in lead times and the potential for delays due to customs. Collaborating with a logistics provider familiar with your region can help streamline the process and mitigate risks associated with international trade. -
How can I vet suppliers of size reduction equipment to ensure quality and reliability?
To vet suppliers, conduct thorough research by reviewing their industry reputation, certifications, and customer testimonials. Request references from other pharmaceutical companies and inquire about their experiences with the supplier’s equipment and service. Additionally, consider visiting the supplier’s facility if possible to inspect their manufacturing processes and quality control measures. It is also beneficial to assess their after-sales support, including maintenance services and availability of spare parts, to ensure long-term reliability. -
What customization options are available for size reduction equipment in the pharmaceutical sector?
Many manufacturers offer customization options for size reduction equipment to meet specific industry needs. Customizations can include modifications to the equipment’s design, materials used, and operational features to accommodate unique materials or production processes. Discuss your requirements with potential suppliers to determine available options, such as adjusting feed sizes, final particle size specifications, and automation features. Tailored solutions can enhance operational efficiency and ensure compliance with industry standards. -
What are the minimum order quantities (MOQs) typically required for size reduction equipment?
Minimum order quantities for size reduction equipment can vary significantly by supplier and equipment type. Some manufacturers may have low MOQs for standard models, while custom solutions might require larger orders to justify production costs. It is advisable to communicate your needs clearly with potential suppliers and negotiate MOQs based on your operational scale. Additionally, consider exploring leasing options or second-hand equipment to reduce initial investment costs, especially for smaller operations. -
What payment terms should I expect when purchasing size reduction equipment internationally?
Payment terms for international purchases can vary widely and typically include options such as advance payment, letters of credit, or payment upon delivery. It’s essential to clarify these terms upfront with your supplier to avoid misunderstandings. Negotiating favorable terms can help manage cash flow, especially for larger capital expenditures. Consider using escrow services for added security in transactions. Additionally, inquire about any available financing options that may ease the financial burden of acquiring new equipment. -
How do I ensure quality assurance (QA) for size reduction equipment in my pharmaceutical facility?
Implementing a robust quality assurance program is critical when integrating size reduction equipment into your pharmaceutical processes. Begin by establishing standard operating procedures (SOPs) for equipment operation, maintenance, and calibration. Regularly conduct inspections and performance evaluations to ensure the equipment meets required specifications. Collaborate with your supplier to understand their QA processes and request documentation proving compliance with industry standards. Additionally, consider third-party audits to verify equipment performance and adherence to regulatory guidelines.
Top 8 Size Reduction Equipment For Pharmaceutical Industry Manufacturers & Suppliers List
1. IQS Directory – Size Reduction Equipment
Domain: iqsdirectory.com
Registered: 2004 (21 years)
Introduction: Size Reduction Equipment: Devices engineered to crush and grind materials, effectively reducing their size. Types include pulverizers, crushers, and grinding media. Applications span various industries such as mining, mineral processing, chemical production, food and beverage, pharmaceuticals, and waste recycling. Key materials handled include coal, shale, brick, concrete, wood, limestone, and pla…
2. Retsch – Size Reduction Equipment
Domain: retsch.com
Registered: 1997 (28 years)
Introduction: Size reduction equipment refers to machinery designed to break solid materials into smaller pieces or finer particles, crucial in industries like mining, food processing, pharmaceuticals, recycling, and chemical manufacturing. Main types include: 1. Crushers: Heavy-duty machines for breaking large chunks into smaller particles (e.g., jaw crushers). 2. Grinders: Reduce materials to a finer size tha…
3. Fitzpatrick – Particle Processing Technologies
Domain: fitzpatrick-mpt.com
Registered: 2019 (6 years)
Introduction: Fitzpatrick offers a range of particle processing and size reduction technologies, including: 1. Hammer Mills: M5A & D6A, DASO6 & DKASO12 2. Scalable Lab System (SLS) 3. SDx™ Series Roller Compaction 4. High Containment Contained System 5. Lab Compaction System. The company specializes in dry granulation and precision particle size reduction, serving industries such as pharmaceutical, nutraceutica…
4. Quadro Engineering – Particle Size Reduction Solutions
Domain: quadro-mpt.com
Registered: 2019 (6 years)
Introduction: Quadro Engineering specializes in particle size reduction and milling equipment with over 40 years of expertise. Key products include: 1. Scalable Lab System (SLS) 2. SDx™ Series – Production-Scale 3. SDx™ Series – Mid-Size 4. Underdriven Comil 5. Overdriven Comil 6. Fine Grinding Solutions 7. Delumping & Screening with FlexSift Technology. The equipment is designed for various applications includ…
5. Microfluidics MPT – Microfluidizer® Technology
Domain: microfluidics-mpt.com
Registered: 2019 (6 years)
Introduction: Microfluidizer® technology for particle size reduction achieves uniform target nanoparticle sizes through high shear processing. Key features include:
– High-pressure pump generating forces up to 30,000 psi (2069 bar)
– Fixed-geometry Interaction Chamber™ for consistent shear levels
– Achieves particle sizes typically 50% smaller than conventional methods
– Benefits include improved bioavailab…
6. Scott Equipment – Size Reduction Solutions
Domain: scottequipment.com
Registered: 1996 (29 years)
Introduction: Scott Equipment specializes in size reduction equipment for various industries, including food, chemical, pharmaceutical, agricultural, and waste reduction. Their product offerings include: 1. **Grinders** 2. **Shredders** 3. **Industrial Crushers** 4. **Atritor Cell Mill** Each machine is custom tailored for specific processes and built durably to withstand demanding applications. The company emp…
7. Kason Corporation – MARION Lump Breakers and Cone Mills
Domain: kason.com
Registered: 1996 (29 years)
Introduction: Kason Corporation supplies and manufactures size reduction screening equipment for various applications, providing high-capacity grinding and milling while minimizing noise, dust, and heat generation. Key products include MARION Lump Breakers and Cone Mills, designed to reduce agglomerates and process sensitive materials at high rates. Equipment features include easily changeable grinding media, l…
8. Hanningfield – Sanitary Milling Equipment
Domain: hanningfield.com
Registered: 2000 (25 years)
Introduction: Pharma Milling Equipment: Hanningfield designs and manufactures sanitary size reduction milling machines for dry and wet processes. The equipment achieves uniform size reduction for applications from R&D to full-scale production, enhancing surface area, bioavailability, dosage uniformity, and formulation dissolution properties. Key products include: Uni-Mill M05-U (low capacity, fast tooling chang…
Strategic Sourcing Conclusion and Outlook for size reduction equipment for pharmaceutical industry
What Are the Key Takeaways for Sourcing Size Reduction Equipment in Pharmaceuticals?
In the pharmaceutical industry, strategic sourcing of size reduction equipment is critical for optimizing production efficiency and ensuring product quality. The diverse range of equipment—from crushers and grinders to pulverizers—enables manufacturers to tailor their processes according to specific material properties and desired particle sizes. Understanding the intricacies of each type of equipment allows buyers to make informed decisions that enhance operational effectiveness and reduce costs.
Why Is Strategic Sourcing Important for B2B Buyers?
Effective strategic sourcing not only secures high-quality equipment but also fosters long-term supplier relationships that can yield significant advantages, including improved technology access and better pricing structures. For international buyers, particularly in emerging markets such as Africa, South America, the Middle East, and Europe, leveraging global supply chains can result in enhanced capabilities and competitive advantages.

Illustrative image related to size reduction equipment for pharmaceutical industry
How Can You Prepare for Future Needs in Size Reduction Equipment?
As the pharmaceutical sector evolves, buyers should remain vigilant regarding technological advancements and regulatory changes that impact equipment performance and compliance. By investing in cutting-edge size reduction technologies now, businesses can future-proof their operations against market shifts and emerging challenges. Engage with suppliers who demonstrate innovative solutions and a commitment to sustainability, positioning your company as a leader in the industry. Take action today to ensure your sourcing strategies align with the future of pharmaceutical manufacturing.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.