A Deep Dive into Tris Lean Solution
Introduction: Navigating the Global Market for tris lean
In today’s competitive landscape, sourcing effective therapeutic solutions such as tris lean presents a significant challenge for international B2B buyers, particularly in regions like Africa, South America, the Middle East, and Europe. With the growing prevalence of conditions requiring innovative treatments, understanding the nuances of tris lean—its applications, types, and market dynamics—becomes essential for informed purchasing decisions. This guide is designed to equip buyers with a thorough understanding of tris lean, covering essential factors such as supplier vetting processes, pricing structures, and regulatory considerations.
Our comprehensive exploration delves into the various forms of tris lean, the latest advancements in formulation technologies, and their therapeutic applications across multiple markets. By highlighting key players and emerging trends, we aim to empower buyers to navigate the complexities of sourcing tris lean effectively. Moreover, this guide addresses critical considerations for establishing reliable partnerships with suppliers, ensuring that your organization can meet patient needs while maintaining compliance with regional regulations.
Ultimately, this resource serves as a vital tool for B2B buyers seeking to optimize their procurement strategies for tris lean, fostering improved patient outcomes and business success. Whether you are in Vietnam or Nigeria, the insights provided here will enhance your ability to make strategic decisions that align with your organization’s goals.
Understanding tris lean Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Extended-Release Liquid | Smooth, consistent medication release; tailored dosing options | ADHD treatment in pediatric and adult populations | Pros: Flexible dosing; easier for patients to consume. Cons: Potential for misuse; requires careful monitoring. |
| Extended-Release Tablet | Solid form with high bioavailability; designed for adult use | ADHD and chronic pain management | Pros: Convenient; longer shelf life. Cons: Less flexible dosing compared to liquids; potential for abuse. |
| Combination Therapy | Uses multiple active ingredients for synergistic effects | Complex conditions requiring multi-faceted treatment | Pros: Addresses multiple symptoms; potentially reduces pill burden. Cons: Increased complexity in prescribing and monitoring. |
| Abuse-Deterrent Formulation | Specially designed to minimize misuse potential | Pain management and ADHD therapies | Pros: Safer for patients; reduces risk of addiction. Cons: May have higher costs; limited availability. |
| Pediatric Formulation | Specifically designed for children; often in liquid form | Treatment of ADHD and other conditions in children | Pros: Tailored for younger patients; better acceptance. Cons: May require more frequent dosing; potential taste issues. |
What Are the Characteristics of Extended-Release Liquid Formulations?
Extended-release liquid formulations are characterized by their smooth and consistent release of medication, making them especially suitable for patients with varying absorption rates. They are commonly used in ADHD treatment, providing a flexible dosing option that can be tailored to individual patient needs. B2B buyers should consider the ease of administration and the potential for patient adherence, but they must also remain vigilant regarding the risk of misuse associated with CNS stimulants.
How Do Extended-Release Tablets Differ in Application?
Extended-release tablets are designed for optimal bioavailability and are primarily targeted at adult patients. They are effective for conditions like ADHD and chronic pain management, offering a convenient option for daily use. When purchasing these products, B2B buyers should weigh the benefits of longer shelf life and ease of storage against the less flexible dosing options compared to liquid forms, which may not suit all patient needs.
What Are the Benefits of Combination Therapies?
Combination therapies leverage multiple active ingredients to provide synergistic effects, particularly beneficial for complex conditions requiring multifaceted treatment approaches. B2B buyers should consider the potential to address a wider range of symptoms with fewer medications, thus reducing the pill burden for patients. However, the complexity in prescribing and monitoring these therapies can pose challenges, necessitating thorough training for healthcare providers.
Why Are Abuse-Deterrent Formulations Important?
Abuse-deterrent formulations are specifically engineered to minimize the potential for misuse and addiction, making them crucial in pain management and ADHD therapies. For B2B buyers, these products offer a safer option for patients at risk of substance abuse. However, they may come at a higher cost and could have limited availability, necessitating careful evaluation of their market presence and pricing structures.
What Should Buyers Consider When Choosing Pediatric Formulations?
Pediatric formulations are tailored specifically for children, often available in liquid form to enhance palatability and ease of administration. These products are essential for treating ADHD and other childhood conditions. B2B buyers should prioritize formulations that ensure better acceptance among young patients, but they must also consider the potential challenges related to frequent dosing and taste preferences, which could impact treatment adherence.
Key Industrial Applications of tris lean
| Industry/Sector | Specific Application of tris lean | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Pharmaceuticals | Development of ADHD medications using LiquiXR® technology | Enhanced patient adherence and satisfaction through consistent delivery | Regulatory compliance, quality assurance, and supply chain reliability |
| Healthcare | Pain management solutions in clinical settings | Improved patient outcomes and reduced side effects | Proven efficacy, safety profile, and market access strategies |
| Addiction Treatment | Formulation of medications for addiction recovery | Addressing unmet needs in patient care | Clinical trial data, long-term stability, and abuse potential monitoring |
| Consumer Health Products | Creation of over-the-counter (OTC) therapeutic options | Broadening market reach and increasing accessibility | Consumer demand trends, packaging, and labeling requirements |
| Research & Development | Investigational therapies for CNS disorders | Pioneering new treatment avenues and expanding product lines | Collaboration with research institutions and funding sources |
How is tris lean Used in Pharmaceuticals for ADHD Medications?
In the pharmaceutical sector, tris lean is pivotal in developing ADHD medications leveraging LiquiXR® technology. This approach enables the creation of extended-release formulations that provide a smooth and consistent delivery of active ingredients. For international B2B buyers, particularly in regions like Africa and South America, sourcing such innovative therapies can significantly improve patient adherence and satisfaction, addressing the global need for effective ADHD treatments. Buyers should prioritize regulatory compliance and robust quality assurance processes to ensure product efficacy and safety.
What Role Does tris lean Play in Healthcare for Pain Management?
Within healthcare, tris lean is utilized to formulate advanced pain management solutions. By incorporating unique mechanisms of action, these medications can reduce side effects and enhance overall patient outcomes. For businesses in the Middle East and Europe, investing in these innovative therapies can not only improve treatment protocols but also meet the growing demand for effective pain relief options. Key considerations for sourcing include the proven efficacy of the products, comprehensive safety profiles, and strategies for market access.
How is tris lean Applied in Addiction Treatment?
Tris lean is increasingly being recognized for its role in formulating medications aimed at addiction recovery. These products address the complex needs of patients struggling with substance use disorders, providing effective treatment alternatives. For B2B buyers in regions like Nigeria and Vietnam, the focus should be on sourcing clinically validated therapies that demonstrate long-term stability and a low potential for abuse. Additionally, understanding the regulatory landscape and ensuring proper monitoring of drug safety is crucial for successful implementation.
What Benefits Does tris lean Offer in Consumer Health Products?
In the consumer health sector, tris lean facilitates the development of over-the-counter (OTC) therapeutic options. This broadens market reach and increases accessibility for consumers seeking effective health solutions. Businesses looking to source these products should consider current consumer demand trends, as well as packaging and labeling requirements that comply with regional regulations. This approach not only enhances product visibility but also aligns with the needs of health-conscious consumers across diverse markets.
How Does tris lean Support Research and Development Efforts?
Tris lean plays a vital role in research and development, particularly for investigational therapies targeting central nervous system disorders. By leveraging innovative technologies, companies can pioneer new treatment avenues and expand their product lines. For international buyers, collaboration with research institutions and securing funding sources are essential for advancing these projects. Furthermore, having access to comprehensive clinical trial data can significantly influence sourcing decisions and foster partnerships in the pharmaceutical landscape.
3 Common User Pain Points for ‘tris lean’ & Their Solutions
Scenario 1: Navigating Regulatory Compliance Challenges with Tris Lean Products
The Problem: B2B buyers often struggle with understanding the complex regulatory landscape surrounding pharmaceutical products, especially when sourcing innovative treatments like those offered by Tris Pharma. This is particularly challenging in regions such as Africa and South America, where regulatory frameworks may differ significantly from Western standards. Buyers may face delays in product approval, unexpected compliance costs, or even the risk of penalties if they misinterpret regulations. This can lead to uncertainty in supply chain management and ultimately affect patient care.
The Solution: To effectively navigate regulatory compliance, buyers should engage with local regulatory experts who understand the nuances of their specific markets. Establish partnerships with legal consultants or regulatory affairs professionals who specialize in pharmaceutical regulations. It’s also crucial to maintain open communication with Tris Pharma representatives to ensure a thorough understanding of product specifications and compliance documentation. Buyers should prioritize sourcing products that have already received regulatory approval in their target regions, as this can mitigate risks associated with compliance. Additionally, leveraging digital platforms that provide real-time updates on regulatory changes can be invaluable for staying informed and proactive.
Scenario 2: Managing Supply Chain Disruptions in Pharmaceutical Distribution
The Problem: B2B buyers often encounter supply chain disruptions that affect the timely delivery of Tris lean products, such as ADHD therapies and CNS disorder treatments. Global events, such as pandemics or geopolitical tensions, can lead to shortages, increased lead times, and fluctuating prices. These disruptions can hinder the ability to provide consistent patient care, especially in areas where access to medications is already limited.
The Solution: To combat supply chain disruptions, buyers should diversify their sourcing strategies by establishing relationships with multiple suppliers. This ensures that if one supplier faces challenges, alternatives are readily available. Implementing inventory management systems that allow for real-time tracking and forecasting can help anticipate shortages and adjust orders accordingly. Additionally, consider utilizing local distributors who have a better understanding of regional logistics and can navigate local challenges more effectively. Building strong relationships with suppliers like Tris Pharma can also facilitate better communication regarding potential disruptions, allowing buyers to plan proactively.
Scenario 3: Addressing Patient-Centric Needs with Customized Treatment Options
The Problem: B2B buyers in healthcare frequently face the challenge of meeting diverse patient needs, particularly in managing conditions like ADHD. Patients may require tailored treatment regimens that account for varying responses to medications, which can be difficult to address with standard pharmaceutical offerings. This variability can lead to dissatisfaction among patients and healthcare providers if their specific needs are not met.
The Solution: To effectively address patient-centric needs, buyers should prioritize products that offer flexible dosing options and delivery forms, such as those developed using Tris Pharma’s LiquiXR® technology. This technology allows for extended-release formulations that can be tailored to individual patient requirements. Buyers should collaborate with healthcare providers to gather feedback on patient experiences and treatment outcomes, which can inform purchasing decisions. Additionally, training sessions with Tris Pharma can equip buyers and healthcare professionals with the knowledge to utilize these innovative therapies effectively, ensuring that they are well-positioned to meet the varied needs of patients. Engaging with patient advocacy groups can also provide insights into emerging needs and preferences, enabling buyers to stay ahead of market demands.
Strategic Material Selection Guide for tris lean
What Are the Key Materials for tris lean and Their Properties?
When selecting materials for tris lean applications, it is crucial to consider properties that directly influence product performance, durability, and compliance with international standards. Below are analyses of four common materials used in tris lean products, focusing on their key properties, advantages, disadvantages, and specific considerations for international B2B buyers.
How Does Polypropylene Perform in tris lean Applications?
Polypropylene (PP) is a thermoplastic polymer known for its excellent chemical resistance and versatility. It can withstand temperatures up to 100°C (212°F) and is resistant to a variety of solvents, making it suitable for tris lean applications that may involve exposure to various chemicals.
Pros: Polypropylene is lightweight, cost-effective, and has good impact resistance. Its manufacturing process is relatively straightforward, which can lower production costs.
Cons: While it has good chemical resistance, it can become brittle at low temperatures and may not be suitable for high-pressure applications.
Impact on Application: Polypropylene’s compatibility with a range of media makes it a preferred choice for many tris lean applications, particularly in environments where chemical exposure is a concern.
Considerations for International Buyers: Buyers should ensure compliance with international standards such as ASTM and ISO. In regions like Africa and South America, sourcing from local suppliers can help mitigate shipping costs and delays.
Why Is Stainless Steel a Preferred Material for tris lean?
Stainless steel, particularly grades 304 and 316, is widely used in tris lean applications due to its superior corrosion resistance and mechanical strength. It can withstand high temperatures and pressures, making it ideal for demanding environments.
Pros: Stainless steel offers excellent durability and longevity, making it a cost-effective choice in the long run. It is also easy to clean and sterilize, which is critical in many medical and pharmaceutical applications.
Cons: The initial cost of stainless steel is higher than other materials like polypropylene. Its manufacturing process can be more complex, potentially leading to longer lead times.
Impact on Application: Stainless steel is highly compatible with various media, including corrosive substances, which enhances its suitability for tris lean products.
Considerations for International Buyers: Buyers must consider compliance with standards like ASTM A240 for stainless steel. In Europe, the EN 10088 standard is also relevant. Understanding local regulations regarding material specifications is essential for buyers in the Middle East and Africa.
What Role Does Glass Play in tris lean Products?
Glass is often used in tris lean applications where transparency and chemical inertness are required. It can handle a wide temperature range and is resistant to many chemicals, making it a viable option for specific applications.
Pros: Glass is non-reactive, ensuring that it does not interfere with the contents it holds. It is also recyclable, which can be an advantage for environmentally conscious companies.
Cons: Glass is heavier and more fragile than other materials, which can complicate handling and transportation. It may also require specialized manufacturing techniques, increasing costs.
Impact on Application: The inert nature of glass makes it suitable for sensitive applications, particularly in pharmaceuticals and food industries.
Considerations for International Buyers: Compliance with standards such as ASTM E438 for glass containers is important. Buyers should also consider the availability of glass in their local markets, as it may not be as readily accessible in some regions.
How Does Polyethylene Compare in tris lean Applications?
Polyethylene (PE) is another thermoplastic that is commonly used in tris lean applications. It is known for its flexibility and resistance to impact and chemicals.
Pros: Polyethylene is lightweight and cost-effective, making it a popular choice for various applications. It can also be produced in various densities, allowing for tailored properties.
Cons: While it has good chemical resistance, it may not perform well under high temperatures or pressures, limiting its use in certain tris lean applications.
Impact on Application: Polyethylene’s compatibility with many substances makes it suitable for various tris lean applications, particularly in packaging and storage.
Considerations for International Buyers: Buyers should ensure that the polyethylene used meets relevant standards, such as ASTM D4976. Understanding local preferences for material types can also influence sourcing decisions.
Summary Table of Material Selection for tris lean
| Material | Typical Use Case for tris lean | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Polypropylene | Chemical containers and packaging | Lightweight and cost-effective | Brittle at low temperatures | Low |
| Stainless Steel | Medical devices and high-pressure applications | Excellent durability and corrosion resistance | Higher initial cost | High |
| Glass | Pharmaceutical vials and sensitive applications | Non-reactive and recyclable | Heavier and more fragile | Medium |
| Polyethylene | Packaging and storage solutions | Flexible and cost-effective | Limited high-temperature performance | Low |
This strategic material selection guide provides valuable insights for international B2B buyers looking to optimize their sourcing strategies for tris lean applications, ensuring compliance and performance across diverse markets.
In-depth Look: Manufacturing Processes and Quality Assurance for tris lean
What Are the Key Stages in the Manufacturing Process of Tris Lean?
The manufacturing process for Tris Lean involves several critical stages that ensure the final product meets high-quality standards. These stages include material preparation, forming, assembly, and finishing.
Material Preparation: How Is It Done?
Material preparation is the foundational step in manufacturing Tris Lean products. This stage involves sourcing high-quality raw materials, which are then subjected to thorough inspections to ensure they meet established specifications. Suppliers must provide certificates of analysis (CoA) to validate the quality and compliance of the raw materials used. This documentation is crucial for B2B buyers, as it provides transparency regarding the materials that will ultimately affect the product’s efficacy and safety.
What Techniques Are Used During the Forming Stage?
The forming stage utilizes advanced techniques to create the desired product shapes and sizes. For Tris Lean, this often involves specialized mixing and granulation methods, particularly when producing oral suspension forms. Technologies such as LiquiXR® are leveraged to ensure a consistent release profile for medications. This proprietary technology allows for precise control over the release rates, enhancing patient adherence and therapeutic outcomes. B2B buyers should inquire about the specific technologies employed during this stage to understand how they contribute to product differentiation.
How Is Assembly Conducted in Tris Lean Manufacturing?
Once the forming stage is complete, the assembly process begins. This stage may involve combining various components, such as active pharmaceutical ingredients (APIs) with excipients, to create the final dosage form. Automated systems are often used to ensure accuracy and efficiency, minimizing the risk of human error. During assembly, rigorous protocols are followed to maintain a sterile environment, especially for products that are sensitive to contamination. B2B buyers should verify that the manufacturer adheres to good manufacturing practices (GMP) during assembly to ensure product integrity.
What Finishing Processes Are Essential for Quality?
Finishing processes play a crucial role in the final product’s appearance and functionality. This stage includes coating, packaging, and labeling. Each of these components must comply with international regulations and standards. For Tris Lean, ensuring that the packaging is tamper-proof and provides adequate protection against environmental factors is vital for maintaining product quality. B2B buyers should look for suppliers who can demonstrate compliance with relevant packaging standards and who conduct thorough testing of their finished products.
What Quality Control Measures Are Implemented in Tris Lean Manufacturing?
Quality control (QC) is an integral part of the manufacturing process for Tris Lean, ensuring that every batch meets stringent quality standards. Various checkpoints and testing methods are employed throughout the manufacturing process.
Which International Standards Govern Quality Control for Tris Lean?
Tris Lean manufacturing adheres to several international quality standards, including ISO 9001 and industry-specific certifications such as CE marking and Good Manufacturing Practices (GMP). ISO 9001 focuses on quality management systems, ensuring that processes consistently produce products that meet customer and regulatory requirements. Compliance with these standards not only enhances product reliability but also builds trust with B2B buyers across different regions.
What Are the Key QC Checkpoints in the Manufacturing Process?
Quality control checkpoints are strategically placed at various stages of the manufacturing process to ensure compliance with established standards. The three primary checkpoints include:
-
Incoming Quality Control (IQC): This involves the inspection of raw materials upon arrival at the manufacturing facility. It ensures that materials meet specified quality criteria before they enter the production process.
-
In-Process Quality Control (IPQC): During the manufacturing process, IPQC monitors various parameters such as temperature, humidity, and equipment performance. This ongoing assessment helps identify and rectify any deviations from quality standards in real-time.
-
Final Quality Control (FQC): After the product is finished, FQC includes comprehensive testing to verify that the final product meets all specifications. This may involve stability testing, potency assays, and microbiological testing.
What Common Testing Methods Are Used in Quality Control?
Several testing methods are commonly employed in the QC process for Tris Lean. These include:
-
Chromatography: Used for analyzing the composition and concentration of active ingredients.
-
Dissolution Testing: Measures the rate and extent to which the active ingredient is released from the product.
-
Microbial Testing: Ensures that the product is free from harmful microorganisms.
-
Stability Testing: Assesses how the product performs under various environmental conditions over time.
B2B buyers should inquire about the specific testing methods employed by their suppliers to ensure that they meet the required safety and efficacy standards.
How Can B2B Buyers Verify Supplier Quality Control?
Verifying a supplier’s quality control processes is essential for B2B buyers to ensure that they are partnering with a reputable manufacturer. Here are some actionable strategies:
What Role Do Audits Play in Supplier Verification?
Regular audits are a critical component of supplier verification. Buyers should conduct both announced and unannounced audits of their suppliers to assess compliance with quality standards and manufacturing practices. These audits can reveal insights into the operational integrity of the supplier, including adherence to GMP and ISO standards.
How Important Are Quality Control Reports?
Requesting detailed quality control reports from suppliers is another effective way to verify their QC processes. These reports should include data on IQC, IPQC, and FQC, highlighting any issues encountered and corrective actions taken. Transparent reporting fosters trust and can help B2B buyers make informed decisions.
Should Buyers Consider Third-Party Inspections?
Engaging third-party inspection services can provide an additional layer of assurance regarding supplier quality. These independent assessments can validate the manufacturer’s claims about their quality processes and product compliance. For international buyers, particularly those from Africa, South America, the Middle East, and Europe, using third-party inspections can mitigate risks associated with cross-border procurement.
What Are the Quality Control and Certification Nuances for International Buyers?
B2B buyers from different regions must be aware of specific nuances regarding quality control and certification. Regulatory requirements can vary significantly between countries, and understanding these differences is crucial for successful international trade.
How Do Regional Regulations Affect Quality Control?
For instance, buyers from Africa may face different regulatory landscapes compared to those in Europe or the Middle East. Familiarity with local regulations, such as the African Medicines Agency (AMA) guidelines or the European Medicines Agency (EMA) standards, can help buyers navigate compliance issues effectively.
What Should Buyers Know About Documentation Requirements?
Documentation is another critical area for international buyers. Ensuring that suppliers provide all necessary documentation, including CoAs, GMP certificates, and regulatory approvals, is vital for maintaining compliance with local laws. Buyers should also verify that these documents are up to date and relevant to the specific products being purchased.
In conclusion, understanding the manufacturing processes and quality assurance measures for Tris Lean is essential for B2B buyers looking to ensure product safety, efficacy, and compliance with international standards. By leveraging the insights provided in this guide, buyers can make informed decisions and foster successful partnerships with their suppliers.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘tris lean’
Introduction
This practical sourcing guide aims to assist B2B buyers in procuring ‘tris lean’, a specialized product designed to meet specific therapeutic needs. By following this step-by-step checklist, buyers will ensure they make informed decisions, optimize supplier selection, and ultimately secure high-quality products that meet their organizational requirements.
Step 1: Define Your Technical Specifications
Clearly outlining your technical specifications is vital to ensure that the ‘tris lean’ products you are sourcing meet your organizational needs. Consider the intended use, dosage forms, and any specific regulatory requirements pertinent to your region. This clarity will help streamline your procurement process and align supplier offerings with your expectations.
Step 2: Research Market Demand and Trends
Understanding current market demand and trends in ‘tris lean’ products is essential for making informed sourcing decisions. Analyze sales data, industry reports, and competitor offerings to gauge market dynamics. This research can reveal emerging needs and help you negotiate better terms with suppliers by demonstrating your market insight.
Step 3: Evaluate Potential Suppliers
Before committing to a supplier, thorough evaluation is crucial. Request company profiles, product samples, and references from other buyers within your industry or region. Consider factors such as:
– Experience: Look for suppliers with a proven track record in delivering ‘tris lean’ products.
– Reputation: Check online reviews and industry feedback to assess reliability and quality.
Step 4: Verify Compliance with Regulatory Standards
Ensuring that suppliers comply with local and international regulatory standards is non-negotiable. Verify certifications such as GMP (Good Manufacturing Practices) and any relevant licenses that affirm the quality and safety of their products. This step mitigates risks associated with non-compliance and protects your organization’s reputation.
Step 5: Assess Pricing and Payment Terms
Review pricing structures and payment terms offered by potential suppliers. It’s important to compare costs while considering the total cost of ownership, which includes shipping, handling, and potential import duties. Look for flexible payment options that can accommodate your cash flow needs, ensuring that you can maintain operational efficiency.
Step 6: Establish Clear Communication Channels
Effective communication with your suppliers is critical for a successful partnership. Establish clear channels for discussing product specifications, order status, and addressing any concerns that may arise. Regular updates and open lines of communication can prevent misunderstandings and foster a collaborative relationship.
Step 7: Plan for Ongoing Evaluation and Feedback
Once you have established a supplier relationship, planning for ongoing evaluation is crucial. Regularly review product performance, supplier responsiveness, and delivery timelines. Collect feedback from end-users to identify areas for improvement, ensuring that your sourcing strategy remains aligned with organizational goals and patient needs.
By adhering to this checklist, B2B buyers can navigate the complexities of sourcing ‘tris lean’ effectively, ensuring they secure high-quality products that meet therapeutic demands while fostering strong supplier relationships.
Comprehensive Cost and Pricing Analysis for tris lean Sourcing
What Are the Key Cost Components in Tris Lean Sourcing?
When considering sourcing for tris lean, it’s vital to understand the various cost components that contribute to the overall expenditure. The primary elements include:
- Materials: The cost of raw materials, such as active pharmaceutical ingredients (APIs) and excipients, is a significant factor. Prices can vary based on the quality and supplier reputation.
- Labor: Labor costs encompass both direct manufacturing personnel and indirect support staff. Skilled labor may be necessary for complex processes, particularly in high-quality production environments.
- Manufacturing Overhead: This includes utilities, rent, and equipment maintenance. Efficient manufacturing processes can reduce overhead costs significantly.
- Tooling: Initial tooling costs can be substantial, especially for custom products. Investing in advanced tooling can enhance production efficiency and reduce long-term costs.
- Quality Control (QC): Rigorous QC measures are essential to ensure product safety and efficacy. The costs associated with testing and regulatory compliance must be factored into the overall budget.
- Logistics: Transportation and warehousing costs can fluctuate based on geographic location, shipping methods, and the need for temperature control in pharmaceutical products.
- Margin: Suppliers typically include a profit margin in their pricing structure, influenced by market demand and competition.
How Do Price Influencers Affect Tris Lean Sourcing?
Several factors can influence pricing in tris lean sourcing:
- Volume/MOQ (Minimum Order Quantity): Bulk purchases often lead to lower per-unit costs. Negotiating favorable terms based on projected sales can yield significant savings.
- Specifications and Customization: Custom formulations may incur additional costs due to unique manufacturing processes or specialized materials. Clear communication of specifications can help manage these costs.
- Materials Quality and Certifications: Higher-quality materials typically come at a premium. Certifications (e.g., GMP, ISO) also play a critical role in pricing, as they assure compliance with industry standards.
- Supplier Factors: The supplier’s reputation, location, and production capabilities can impact pricing. Engaging with suppliers who demonstrate reliability and innovation can lead to better pricing and service.
- Incoterms: Understanding Incoterms is crucial for international buyers. They dictate the responsibilities of buyers and sellers regarding transportation costs, risk, and insurance, which can significantly influence total costs.
What Buyer Tips Can Optimize Cost-Efficiency in Tris Lean Sourcing?
B2B buyers can implement several strategies to enhance cost efficiency:
- Negotiation: Always engage in negotiations. Understanding the supplier’s cost structure can provide leverage to secure better pricing or terms.
- Total Cost of Ownership (TCO): Consider not just the purchase price, but the TCO, which includes logistics, storage, and disposal costs. This holistic view can reveal more cost-effective options.
- Pricing Nuances for International Buyers: Buyers from Africa, South America, the Middle East, and Europe should be aware of additional costs such as tariffs, taxes, and currency fluctuations. Building these into the budget can prevent unexpected expenses.
- Long-Term Relationships: Establishing long-term relationships with suppliers can lead to better pricing, improved service, and priority during supply shortages. Regular communication and collaboration can foster a partnership that benefits both parties.
Disclaimer on Pricing
All prices mentioned in this analysis are indicative and may vary based on market conditions, supplier negotiations, and specific buyer requirements. It is advisable for buyers to conduct thorough market research and obtain multiple quotes to ensure competitive pricing.
Alternatives Analysis: Comparing tris lean With Other Solutions
Understanding Alternatives in the B2B Landscape
In the competitive landscape of business solutions, identifying alternatives to established products or methods is crucial for informed decision-making. Buyers must evaluate various options to ensure they choose the solution that best meets their needs. This analysis focuses on comparing ‘Tris Lean’ with other viable alternatives, providing insights into their performance, cost, ease of implementation, maintenance, and ideal use cases.
Comparison Table
| Comparison Aspect | Tris Lean | Alternative 1: Agile Methodology | Alternative 2: Lean Six Sigma |
|---|---|---|---|
| Performance | High efficiency in project execution | Improved adaptability and responsiveness | Enhanced process efficiency with quality control |
| Cost | Moderate investment with potential ROI | Lower upfront costs, but may require ongoing training | Higher initial costs due to training and implementation |
| Ease of Implementation | Requires structured onboarding | Flexible implementation, can be adapted easily | Requires comprehensive training for staff |
| Maintenance | Moderate ongoing support needed | Low maintenance, continuous improvement culture | Regular audits and process assessments necessary |
| Best Use Case | Projects requiring tight control and efficiency | Dynamic environments needing rapid response | Organizations focused on quality and waste reduction |
Detailed Breakdown of Alternatives
Alternative 1: Agile Methodology
The Agile methodology is renowned for its flexibility and speed in project management. This approach allows teams to adapt quickly to changing requirements, making it ideal for dynamic environments. Pros include lower upfront costs and a culture of continuous improvement, which can enhance team morale and engagement. However, the need for ongoing training and the potential for scope creep can pose challenges, particularly in larger organizations where structure is vital.
Alternative 2: Lean Six Sigma
Lean Six Sigma combines the principles of Lean manufacturing and Six Sigma, focusing on waste reduction and quality improvement. This methodology is particularly effective for organizations that prioritize efficiency while maintaining high standards. The comprehensive training required ensures a deep understanding of the processes among staff, leading to sustainable improvements. However, the initial investment in training and the ongoing need for process audits can be a barrier for some companies, especially smaller businesses with limited resources.
Choosing the Right Solution for Your Business Needs
Selecting the most appropriate solution involves understanding your organization’s specific context and goals. For businesses in fast-paced industries, Agile may provide the necessary responsiveness. In contrast, those focused on quality control and process efficiency might find Lean Six Sigma more beneficial. ‘Tris Lean’ offers a robust option for organizations seeking structured project management with a focus on efficiency. Ultimately, a thorough assessment of each alternative’s strengths and weaknesses, in relation to your unique business challenges, will guide you toward the best choice.
Essential Technical Properties and Trade Terminology for tris lean
What Are the Key Technical Properties of Tris Lean?
Understanding the essential technical properties of tris lean is crucial for B2B buyers in sectors such as pharmaceuticals, especially in emerging markets like Africa, South America, and the Middle East. Here are the critical specifications that should guide your purchasing decisions:
1. Material Grade
Material grade refers to the classification of the substances used in manufacturing tris lean products. High-quality grades ensure efficacy and safety, which are paramount in therapeutic applications. Buyers should verify material specifications to ensure compliance with international standards, as this impacts product reliability and performance.
2. Tolerance Levels
Tolerance levels denote the permissible variations in dimensions and performance characteristics of tris lean products. In pharmaceuticals, tight tolerances are essential to maintain dosage accuracy and ensure patient safety. Understanding these levels helps buyers assess product quality and suitability for their specific applications.
3. Release Profile
The release profile indicates how a product, such as a medication, releases its active ingredients over time. For tris lean, which may utilize advanced delivery systems, a smooth and consistent release profile is critical. This characteristic enhances therapeutic effectiveness and patient adherence, making it an essential consideration for procurement.
4. Bioavailability
Bioavailability measures the proportion of an active ingredient that enters systemic circulation when introduced into the body. Higher bioavailability means better therapeutic outcomes. B2B buyers must prioritize products with optimized bioavailability to ensure that their patients receive maximum benefit from the treatments.
5. Stability Data
Stability data provides insight into how well a product retains its efficacy and safety over time under various conditions. For tris lean products, stability is crucial for shelf life and regulatory compliance. Buyers should request stability studies to ensure that the products will remain effective throughout their intended use period.
6. Regulatory Compliance
Regulatory compliance refers to adherence to local and international pharmaceutical regulations governing the manufacturing and distribution of products. Buyers need to ensure that tris lean offerings meet all relevant guidelines to avoid legal complications and ensure patient safety.
What Are Common Trade Terms Related to Tris Lean?
Familiarity with trade terminology is essential for navigating the B2B landscape effectively. Here are several key terms that buyers should know:
1. OEM (Original Equipment Manufacturer)
OEM refers to companies that produce components or products that are then marketed under another company’s brand. In the context of tris lean, understanding OEM relationships can help buyers identify reliable sources of high-quality products tailored to their specifications.
2. MOQ (Minimum Order Quantity)
MOQ is the smallest quantity of a product that a supplier is willing to sell. This term is critical for B2B buyers as it affects inventory management and cost efficiency. Buyers should negotiate MOQs to align with their purchasing capabilities and market demands.
3. RFQ (Request for Quotation)
An RFQ is a document sent to suppliers requesting pricing information for specific products or services. This process is essential for obtaining competitive pricing on tris lean products, allowing buyers to make informed procurement decisions based on cost and quality.
4. Incoterms
Incoterms are international commercial terms that define the responsibilities of buyers and sellers in the shipping process. Familiarity with these terms is vital for B2B transactions, as they clarify shipping costs, risks, and responsibilities, impacting overall procurement strategies.
5. Lead Time
Lead time refers to the amount of time it takes from placing an order until the product is delivered. Understanding lead times for tris lean products helps buyers plan their inventory and ensure timely availability of essential medications, particularly in markets with fluctuating demand.
6. Pharmaceutical Supply Chain
This term encompasses all stages involved in the production and distribution of pharmaceutical products, from raw material sourcing to final delivery. A thorough understanding of the pharmaceutical supply chain for tris lean products enables buyers to optimize procurement strategies and ensure consistent product availability.
By grasping these technical properties and trade terms, B2B buyers can make informed decisions that enhance their procurement processes and ultimately improve patient outcomes.
Navigating Market Dynamics and Sourcing Trends in the tris lean Sector
What Are the Current Market Dynamics and Key Trends in the Tris Lean Sector?
The tris lean sector is witnessing a transformative evolution, driven by several global factors. One of the primary drivers is the rising prevalence of conditions such as ADHD, chronic pain, and addiction, prompting an increased demand for effective therapeutic solutions. As international B2B buyers from regions like Africa, South America, the Middle East, and Europe seek innovative treatments, the focus is shifting towards suppliers that can offer differentiated products with unique delivery mechanisms, such as Tris Pharma’s proprietary LiquiXR® technology.
Emerging B2B tech trends are also shaping the landscape. Digital health solutions, including telemedicine and mobile health applications, are enhancing patient engagement and adherence to treatment regimens. This integration of technology into healthcare is influencing sourcing strategies, as buyers prioritize partnerships with companies that leverage data analytics and digital platforms to optimize supply chains and improve patient outcomes.
Additionally, sustainability and ethical considerations are becoming increasingly important for B2B buyers. Companies are expected to demonstrate their commitment to responsible sourcing and environmental stewardship. This trend is particularly pronounced in European markets, where regulatory frameworks are stringent, and consumers demand transparency in product sourcing.
How Does Sustainability and Ethical Sourcing Impact the Tris Lean Sector?
The environmental impact of pharmaceutical manufacturing is under scrutiny, making sustainability a critical focus for B2B buyers in the tris lean sector. Ethical sourcing practices not only mitigate environmental risks but also enhance brand reputation and customer trust. International buyers are more inclined to partner with suppliers who prioritize sustainable manufacturing processes and adhere to environmental regulations.
‘Green’ certifications and materials are increasingly sought after as companies strive to meet consumer demand for eco-friendly products. Certifications like ISO 14001, which focuses on effective environmental management systems, can serve as a key differentiator in the sourcing process. Buyers should actively seek suppliers who are transparent about their sourcing practices and demonstrate a commitment to reducing their carbon footprint, as these factors can significantly influence purchasing decisions.
Moreover, the emphasis on ethical supply chains extends beyond environmental concerns. Social responsibility, including fair labor practices and community engagement, is becoming a vital criterion for evaluating potential partners. Companies that prioritize ethical sourcing are likely to foster stronger relationships with their clients and enhance their competitive advantage in the market.
What Is the Brief Evolution and History of the Tris Lean Sector?
The tris lean sector has evolved significantly over the past few decades, transitioning from traditional treatment modalities to more innovative and patient-centered approaches. Initially dominated by standard medications, the sector has experienced a paradigm shift with the introduction of advanced drug delivery systems and specialized formulations aimed at enhancing therapeutic efficacy and patient compliance.
The emergence of companies like Tris Pharma has played a crucial role in this evolution. By leveraging proprietary technologies such as LiquiXR®, Tris Pharma has introduced a range of ADHD therapies that address the unique needs of diverse patient populations. This focus on innovation has not only improved patient outcomes but has also set new standards for quality and effectiveness in the industry.
As the market continues to evolve, the emphasis on personalized medicine and tailored therapeutic solutions is expected to grow, further shaping the landscape of the tris lean sector and influencing B2B sourcing strategies on a global scale.
Frequently Asked Questions (FAQs) for B2B Buyers of tris lean
-
How do I solve challenges related to sourcing tris lean products?
Sourcing tris lean products can be complex due to varying regulations and quality standards across regions. To address this, start by identifying reliable suppliers with a proven track record in your target market. Conduct thorough research, including reviews and testimonials, and consider reaching out to industry networks for recommendations. Establish clear communication regarding your specific requirements and expectations. Furthermore, requesting samples for quality assessment can help ensure the products meet your standards before committing to larger orders. -
What is the best strategy for negotiating prices with tris lean suppliers?
To negotiate effectively with tris lean suppliers, conduct market research to understand average pricing and competitor offerings. Approach negotiations with a clear budget and be prepared to discuss volume commitments, which can often lead to better pricing. Emphasize long-term partnerships and potential future orders to encourage suppliers to offer more favorable terms. Consider leveraging trade agreements or local market insights to strengthen your negotiation position. Remember, building a good relationship with suppliers can lead to better terms over time. -
What customization options are available for tris lean products?
Many suppliers of tris lean products offer customization options to meet specific needs, such as formulation adjustments, packaging variations, or dosage modifications. When sourcing, inquire about the supplier’s capabilities for custom solutions, including minimum order quantities for tailored products. Be clear about your requirements and any regulatory considerations that may apply in your region. Engaging in open dialogue with potential suppliers can help you explore innovative options that align with your business objectives. -
What are the minimum order quantities (MOQ) for tris lean products?
Minimum order quantities (MOQ) can vary significantly among suppliers, often depending on the type of tris lean product and production capabilities. Typically, MOQs are established to ensure cost-effectiveness for both the supplier and the buyer. It’s essential to communicate your intended order size during the initial discussions to understand the supplier’s flexibility. If your order volume is lower than their MOQ, inquire about options such as group purchasing or partnering with other businesses to meet the required quantity. -
What payment terms should I expect when sourcing tris lean?
Payment terms for tris lean products can differ based on the supplier and your location. Common arrangements include upfront payments, deposits, or net terms that allow payment after delivery. It’s crucial to discuss payment options early in the negotiation process to ensure both parties are comfortable. Consider using secure payment methods that offer buyer protection, especially when dealing with international suppliers. Establishing clear payment timelines and conditions can help prevent misunderstandings and build trust with your supplier. -
How can I ensure quality assurance (QA) when sourcing tris lean products?
To ensure quality assurance when sourcing tris lean products, it’s vital to partner with suppliers who adhere to industry standards and possess relevant certifications. Request documentation that demonstrates their compliance with Good Manufacturing Practices (GMP) and any other applicable regulations. Conducting site visits or audits, if feasible, can provide deeper insights into their production processes. Additionally, implementing a quality control protocol on your end, including batch testing and inspections, can help maintain product integrity throughout the supply chain. -
What logistics considerations should I keep in mind when importing tris lean products?
When importing tris lean products, logistics considerations include transportation methods, customs regulations, and potential tariffs. Ensure that your supplier has experience with international shipping and can provide necessary documentation for customs clearance. Collaborate with a reliable freight forwarder familiar with your destination country’s import requirements. Additionally, factor in lead times for production and shipping to avoid delays in your supply chain. Proper planning and communication with your logistics partners can help streamline the import process. -
How do I vet potential suppliers of tris lean products for reliability?
To vet potential suppliers for reliability, start by reviewing their credentials, including certifications and industry experience. Look for suppliers with a strong reputation, backed by positive reviews from other B2B buyers. Request references and conduct background checks to assess their financial stability and operational history. Additionally, engage in direct conversations to gauge their responsiveness and willingness to address your concerns. Building relationships with suppliers who prioritize transparency and communication can significantly enhance your sourcing experience.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.
Top 6 Tris Lean Manufacturers & Suppliers List
1. Tris Pharma – ADHD Therapies
Domain: trispharma.com
Registered: 2000 (25 years)
Introduction: Tris Pharma has developed a portfolio of four leading ADHD therapies designed to enhance patient care and experience for both adults and children. They utilize proprietary LiquiXR® technology, which has powered the development of over a dozen medicines and programs. The company is also working on investigational therapies targeting ADHD, pain, addiction, and other CNS disorders, including Cebranop…
2. Tuzistra XR – Codeine-Based Cough Syrup
Domain: europeanpharmaceuticalreview.com
Registered: 2006 (19 years)
Introduction: Tuzistra XR is the only codeine-based extended-release cough cold syrup available to patients and physicians in the United States. Dyanavel XR is an Extended-Release Oral Suspension for ADHD, for which the FDA has accepted a New Drug Application for review.
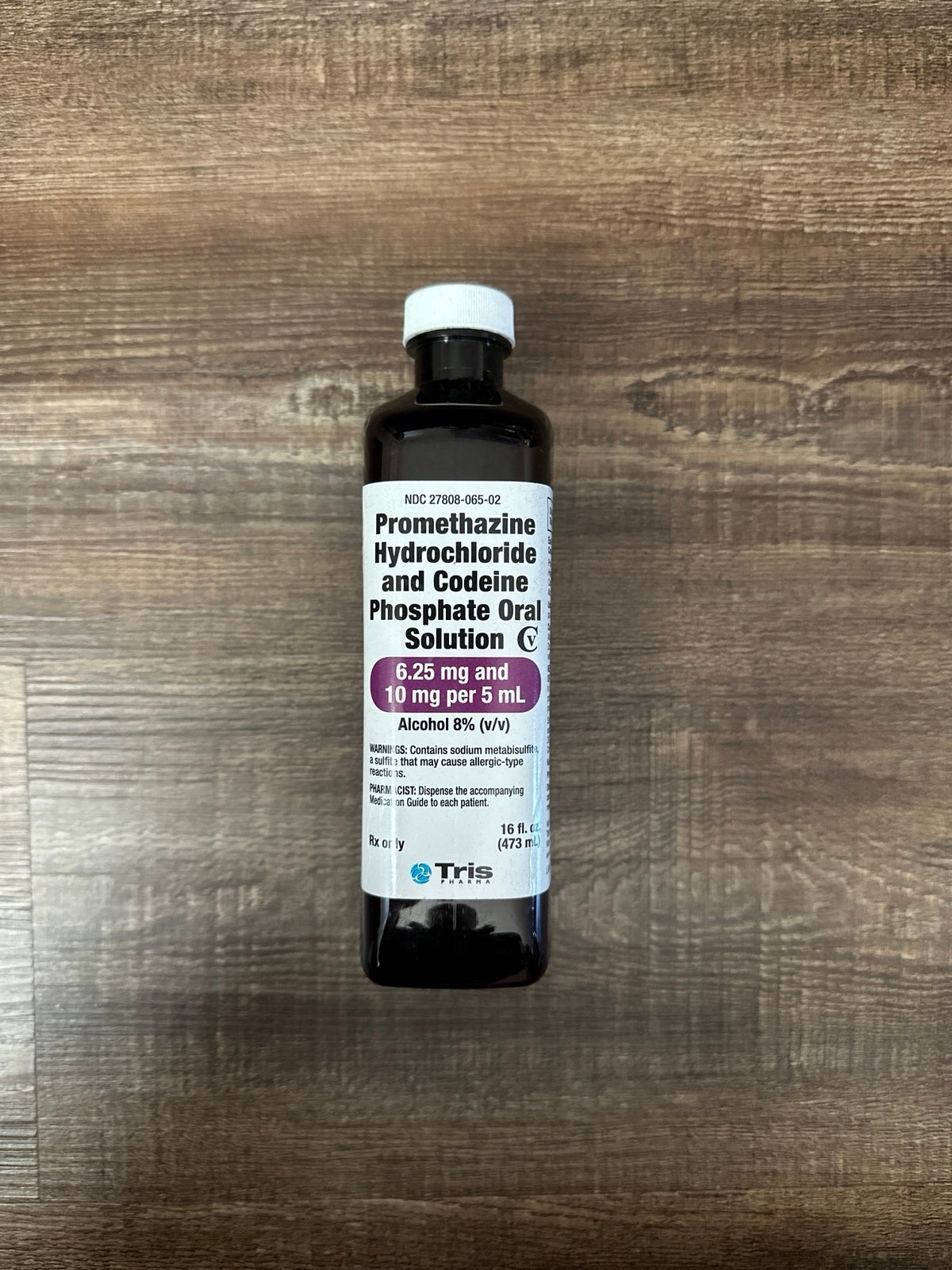
3. Tris Pharma – Promethazine and Codeine Syrup
Domain: drugstorenews.com
Registered: 1995 (30 years)
Introduction: Tris Pharma has launched promethazine and codeine syrup, which is an oral solution containing promethazine HCl and codeine phosphate. The product is manufactured in Monmouth Junction, N.J., and will be available in 16-oz. bottles with dosage strengths of 6.25 mg and 10 mg per 5-ml oral solution. It is indicated for temporary relief of coughs and upper respiratory symptoms associated with allergies…
4. DYANAVEL XR – ADHD Treatment
Domain: trismedical.com
Registered: 2021 (4 years)
Introduction: DYANAVEL XR (amphetamine) is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older. It is available in two dosage forms: an extended-release oral suspension containing 2.5 mg amphetamine base equivalents per mL and extended-release tablets in strengths of 5 mg, 10 mg, 15 mg, and 20 mg. The recommended s…
5. Walgreens – Allergy and Motion Sickness Relief
Domain: walgreens.com
Registered: 1995 (30 years)
Introduction: This company, Walgreens – Allergy and Motion Sickness Relief, is a notable entity in the market. For specific product details, it is recommended to visit their website directly.
6. Promethazine with Codeine – Prescription Cough Relief
Domain: goodrx.com
Registered: 2011 (14 years)
Introduction: Promethazine with Codeine is a prescription-only cough and cold medication that has been discontinued by Actavis due to its popularity for recreational use. It is indicated for the temporary relief of coughs and upper respiratory tract symptoms related to allergies or the common cold. Promethazine is an antihistamine used to treat allergy symptoms, while codeine is an opioid analgesic used for pai…
Strategic Sourcing Conclusion and Outlook for tris lean
In today’s dynamic pharmaceutical landscape, strategic sourcing is paramount for international B2B buyers, especially in regions like Africa, South America, the Middle East, and Europe. Tris Pharma’s commitment to innovation through proprietary technologies such as LiquiXR® provides a unique opportunity for buyers to access differentiated therapeutic solutions for ADHD, pain management, and CNS disorders. By leveraging Tris’s extensive portfolio of patient-friendly medications, buyers can enhance their offerings, ensuring they meet diverse patient needs while maintaining compliance with global health standards.
The emphasis on strategic sourcing not only ensures access to high-quality therapeutics but also fosters partnerships that can drive mutual growth. As the demand for effective and innovative treatments continues to rise, aligning with a forward-thinking manufacturer like Tris Pharma will empower B2B buyers to stay ahead of market trends and patient needs.
Looking ahead, now is the time for international buyers to engage with Tris Pharma. By integrating these innovative solutions into their product lines, they can significantly improve patient outcomes and establish a competitive edge in their respective markets. Explore the potential of strategic partnerships today and be part of the transformation in healthcare delivery.