How to Source Stability Chambers Effectively: A 2025 Checklist
Introduction: Navigating the Global Market for stability chambers
In the ever-evolving landscape of pharmaceuticals and biotechnology, sourcing stability chambers that meet stringent regulatory requirements can be a daunting challenge for international B2B buyers. These critical environments are essential for conducting stability studies, ensuring product efficacy, and maintaining compliance with International Conference on Harmonization (ICH) guidelines. This comprehensive guide delves into the diverse types of stability chambers available, their specific applications across industries, and key considerations for supplier vetting and cost evaluation.
Navigating the global market for stability chambers requires a nuanced understanding of both the technical specifications and the unique regulatory landscapes of various regions, including Africa, South America, the Middle East, and Europe. Buyers from countries like Vietnam and Saudi Arabia will find tailored insights that address their specific needs and challenges. By equipping you with actionable information on chamber features, performance standards, and maintenance requirements, this guide empowers you to make informed purchasing decisions that align with your operational goals.
Whether you are looking for large walk-in chambers for extensive testing or compact units for smaller laboratories, this resource will help you identify the optimal solutions for your organization. Unlock the potential of your research and development efforts by confidently selecting the right stability chamber that ensures product integrity and compliance in a global marketplace.
Understanding stability chambers Types and Variations
| Type Name | Key Distinguishing Features | Primary B2B Applications | Brief Pros & Cons for Buyers |
|---|---|---|---|
| Walk-In Stability Chambers | Large capacity, customizable sizes, and configurations | Pharmaceutical stability testing, bulk product storage | Pros: High capacity, flexible design. Cons: Higher initial cost and space requirements. |
| Benchtop Stability Chambers | Compact design, suitable for smaller labs | Small-scale testing, R&D labs | Pros: Space-efficient, affordable. Cons: Limited capacity and features compared to larger models. |
| Humidity Test Chambers | Specific humidity control features, often with light control | Shelf-life testing for food and pharmaceuticals | Pros: Precise humidity control, ICH compliance. Cons: May require additional water supply or maintenance. |
| Constant Climate Chambers | Stable temperature and humidity, often with light control | Environmental testing, material stability studies | Pros: Reliable performance, versatile applications. Cons: Can be expensive and require calibration. |
| Custom Environmental Rooms | Tailored configurations for specific testing needs | Research institutions, specialized product testing | Pros: Fully customizable, meets unique requirements. Cons: Longer lead times and potentially higher costs. |
What Are Walk-In Stability Chambers and Their Benefits for B2B Buyers?
Walk-in stability chambers are designed for large-scale testing and storage, making them ideal for pharmaceutical companies and manufacturers needing to test bulk products. Their customizable sizes and configurations allow for the accommodation of various products and testing protocols. Buyers should consider the initial investment and space requirements, as these chambers can be costly and require dedicated areas within facilities.
How Do Benchtop Stability Chambers Fit into Small Lab Environments?
Benchtop stability chambers are compact and designed for smaller laboratory environments. They are perfect for R&D labs or companies that conduct small-scale testing. Their affordability and space-efficient design make them attractive to startups or smaller enterprises. However, potential buyers should be aware of their limited capacity and functionality compared to larger walk-in models.
What Makes Humidity Test Chambers Essential for Pharmaceutical Testing?
Humidity test chambers are specifically engineered to control humidity levels, crucial for testing the stability of pharmaceuticals and food products. Many models also incorporate light control features to simulate various environmental conditions. While they offer precise humidity management, buyers need to consider the additional requirements for water supply and maintenance, which can impact overall operational costs.
Why Choose Constant Climate Chambers for Environmental Testing?
Constant climate chambers are built to provide stable temperature and humidity conditions, essential for environmental testing and material stability studies. Their reliability makes them suitable for various industries, including pharmaceuticals and materials science. However, buyers should be prepared for the higher costs and the need for regular calibration to maintain accuracy.
What Are the Advantages of Custom Environmental Rooms for Specialized Testing?
Custom environmental rooms can be tailored to meet specific testing requirements, making them a valuable asset for research institutions and companies with unique product testing needs. Their versatility allows for a wide range of applications, from drug testing to specialized product development. However, the longer lead times and potentially higher costs associated with custom solutions may require careful budgeting and planning.
Key Industrial Applications of stability chambers
| Industry/Sector | Specific Application of Stability Chambers | Value/Benefit for the Business | Key Sourcing Considerations for this Application |
|---|---|---|---|
| Pharmaceuticals | Drug stability testing under ICH guidelines | Ensures product efficacy and safety, facilitating regulatory approval | Compliance with ICH Q1A standards, precise temperature/humidity control |
| Food and Beverage | Shelf-life testing for packaged goods | Validates product quality and extends marketability | Size and capacity to accommodate various product types |
| Biotechnology | Environmental testing for biological samples | Supports research integrity and product development | Customization options for specific research protocols |
| Cosmetics and Personal Care | Stability testing for formulations | Guarantees product stability and consumer safety | Ability to replicate diverse environmental conditions |
| Agricultural Research | Insect hatching and seed viability studies | Enhances research accuracy and agricultural productivity | Specific humidity and temperature ranges for different species |
How Are Stability Chambers Used in the Pharmaceutical Industry?
In the pharmaceutical sector, stability chambers are crucial for conducting drug stability testing, adhering to the International Conference on Harmonization (ICH) guidelines. These chambers provide controlled environments where temperature and humidity can be precisely regulated, ensuring that drugs maintain their efficacy and safety over time. For international buyers, especially in regions like Africa and South America, sourcing chambers that comply with local regulatory standards is essential. Factors such as reliability, energy efficiency, and technical support should also be considered to ensure seamless operations.
Illustrative image related to stability chambers
What Role Do Stability Chambers Play in Food and Beverage Testing?
Stability chambers are instrumental in the food and beverage industry for shelf-life testing. By simulating various environmental conditions, these chambers help manufacturers assess how different factors affect product quality and longevity. This capability is vital for businesses aiming to validate their packaging solutions and extend their products’ marketability. Buyers from the Middle East and Europe should prioritize chambers that offer flexibility in size and configuration to accommodate different packaged goods while ensuring compliance with local food safety regulations.
How Are Stability Chambers Beneficial for Biotechnology Research?
In biotechnology, stability chambers provide a controlled environment for environmental testing of biological samples, such as cultures and cell lines. These chambers ensure that researchers can conduct experiments under consistent conditions, which is critical for obtaining reliable results. International buyers must look for features that allow customization to meet specific research protocols, including varying humidity and temperature ranges. This adaptability is particularly important for regions like Europe, where stringent research standards prevail.
Why Are Stability Chambers Essential for Cosmetics and Personal Care Products?
Stability chambers play a vital role in the cosmetics and personal care industry by testing the stability of various formulations. These chambers help manufacturers ensure that their products remain effective and safe for consumers throughout their shelf life. For buyers in emerging markets, the ability to simulate different environmental conditions is crucial for developing formulations that can withstand diverse climates. Sourcing considerations should include the chamber’s ability to replicate conditions specific to various product types.

Illustrative image related to stability chambers
How Do Stability Chambers Support Agricultural Research?
In agricultural research, stability chambers are utilized for insect hatching and seed viability studies. These controlled environments enable researchers to optimize conditions for growth and development, leading to improved agricultural productivity. Buyers, particularly from regions with diverse agricultural practices, should focus on chambers that provide precise control over temperature and humidity, tailored to the specific needs of different species. This ensures that research findings are valid and applicable to real-world agricultural scenarios.
3 Common User Pain Points for ‘stability chambers’ & Their Solutions
Scenario 1: Navigating Regulatory Compliance Challenges
The Problem: For many B2B buyers, particularly those in pharmaceutical and biotech sectors, ensuring compliance with stringent regulatory standards like the ICH (International Conference on Harmonization) can be daunting. Stability chambers must not only maintain precise temperature and humidity levels but also provide documented evidence of compliance through rigorous testing protocols. Failure to meet these standards can result in costly delays, product recalls, or even legal repercussions, making it critical for buyers to choose the right equipment from the outset.
The Solution: To navigate these regulatory complexities, buyers should prioritize sourcing stability chambers that are explicitly designed to meet ICH guidelines. When evaluating options, inquire about the chamber’s compliance certifications and request temperature mapping and calibration documentation. Additionally, consider manufacturers that provide integrated data logging and reporting features, which can automate compliance documentation and simplify audits. Investing in stability chambers that offer extensive customization options—such as different humidity systems and access ports—can also enhance your ability to meet specific testing protocols and adapt to changing regulatory demands.

Illustrative image related to stability chambers
Scenario 2: Managing Equipment Downtime and Reliability
The Problem: Stability chambers are essential for conducting drug stability studies, shelf-life testing, and other critical applications. However, equipment failure or malfunction can lead to significant downtime, jeopardizing research timelines and product release schedules. This issue is particularly concerning in regions where access to immediate technical support may be limited, leading to extended periods of unproductive time.
The Solution: To mitigate the risk of equipment downtime, buyers should look for stability chambers known for their reliability and robust construction. Choose models with a proven track record of performance under varying environmental conditions. It’s advisable to establish a preventative maintenance schedule with the manufacturer or a local service provider to ensure regular checks and timely repairs. Furthermore, consider investing in a chamber that features advanced diagnostics and alert systems to notify users of potential issues before they escalate into failures. By selecting equipment that comes with strong customer support and warranty options, businesses can safeguard against unexpected disruptions.
Scenario 3: Addressing Space and Accessibility Constraints
The Problem: Many laboratories face spatial limitations that complicate the installation of stability chambers. Buyers may struggle to find chambers that fit within their designated lab spaces while still providing sufficient capacity for their testing needs. Additionally, accessibility concerns, such as door width or weight restrictions, can pose challenges when moving equipment into place.
The Solution: When sourcing stability chambers, it’s essential to assess the physical space available and select models that fit those dimensions. Buyers should request detailed specifications, including exterior dimensions and door configurations, to ensure compatibility with existing lab layouts. Manufacturers that offer modular or stackable designs can provide flexible solutions for space-constrained environments. Additionally, consider options that are designed for easy transport, such as units with rolling capabilities or those that can be disassembled for easier maneuverability. Collaborating with the manufacturer during the planning phase can help identify the best chamber layout and configuration to maximize efficiency while adhering to spatial constraints.
Strategic Material Selection Guide for stability chambers
When selecting materials for stability chambers, it is crucial to consider their properties, advantages, disadvantages, and suitability for specific applications. The following analysis highlights four common materials used in the construction of stability chambers, providing actionable insights for international B2B buyers.
What Are the Key Properties of Stainless Steel for Stability Chambers?
Stainless steel is a widely utilized material in the manufacturing of stability chambers due to its excellent mechanical properties and resistance to corrosion. It typically has a temperature rating that can withstand extreme conditions, making it suitable for environments ranging from -40°C to 70°C. Its non-reactive nature ensures that it does not leach chemicals into the chamber, preserving the integrity of the samples stored within.
Pros: Stainless steel is highly durable and easy to clean, making it ideal for sterile environments. It also has a long lifespan, which reduces the need for frequent replacements.
Cons: The primary drawback is its cost, which can be significantly higher than other materials. Additionally, manufacturing processes can be complex, leading to longer lead times.
Impact on Application: Stainless steel is compatible with a wide range of media, including biological samples and pharmaceuticals, ensuring that it meets the stringent requirements of ICH guidelines.

Illustrative image related to stability chambers
Considerations for International Buyers: Buyers from regions such as Africa and South America should ensure compliance with international standards like ASTM and DIN. The availability of stainless steel may vary, so sourcing from local suppliers could help mitigate import costs.
How Does Polycarbonate Compare as a Material for Stability Chambers?
Polycarbonate is another popular choice for stability chambers, particularly for applications requiring visibility. This thermoplastic material is known for its high impact resistance and good thermal insulation properties, withstanding temperatures from -40°C to 120°C.
Pros: Polycarbonate is lightweight, making it easier to handle and install. Its transparency allows for easy monitoring of samples without opening the chamber.
Cons: While it is resistant to breakage, polycarbonate can be susceptible to scratching and may degrade under prolonged exposure to UV light.

Illustrative image related to stability chambers
Impact on Application: Polycarbonate is suitable for chambers that require frequent monitoring, particularly in research environments focusing on biological studies.
Considerations for International Buyers: Buyers should consider the UV stability of polycarbonate in regions with high sunlight exposure, such as the Middle East. Compliance with local regulations regarding plastic materials is also essential.
What Are the Benefits of Glass in Stability Chambers?
Glass is often used in stability chambers for its inert properties and excellent visibility. It can withstand a wide range of temperatures and provides a high level of chemical resistance, making it suitable for various applications.
Pros: Glass is non-reactive, ensuring that no contaminants leach into the chamber. Its transparency allows for easy observation of the contents.
Cons: The fragility of glass can be a significant drawback, as it is prone to breakage. Additionally, glass chambers can be heavier and more challenging to transport.

Illustrative image related to stability chambers
Impact on Application: Glass is particularly beneficial in applications where sample integrity is critical, such as in pharmaceutical testing.
Considerations for International Buyers: Buyers should ensure that glass meets local safety standards, especially in regions prone to seismic activity. Import duties on glass products may also affect overall costs.
How Does Aluminum Perform as a Material for Stability Chambers?
Aluminum is a lightweight metal often used in the construction of stability chambers due to its excellent thermal conductivity and resistance to corrosion. It typically operates effectively within a temperature range of -20°C to 80°C.
Pros: Aluminum is cost-effective compared to stainless steel and is easier to fabricate, which can reduce manufacturing lead times.
Cons: While it is resistant to corrosion, aluminum can be less durable than stainless steel and may require more frequent maintenance.
Impact on Application: Aluminum is suitable for less stringent applications where cost is a major concern, such as in educational institutions or smaller laboratories.
Considerations for International Buyers: Buyers should evaluate the local availability of aluminum and its compliance with international standards. In regions with high humidity, additional coatings may be necessary to enhance corrosion resistance.

Illustrative image related to stability chambers
Summary Table of Material Selection for Stability Chambers
| Material | Typical Use Case for stability chambers | Key Advantage | Key Disadvantage/Limitation | Relative Cost (Low/Med/High) |
|---|---|---|---|---|
| Stainless Steel | Pharmaceutical storage, biological samples | High durability and corrosion resistance | Higher cost and complex manufacturing | High |
| Polycarbonate | Research environments requiring visibility | Lightweight and easy to monitor | Susceptible to scratches and UV damage | Medium |
| Glass | Pharmaceutical testing, sample integrity | Non-reactive and excellent visibility | Fragile and heavy | Medium |
| Aluminum | Educational institutions, smaller labs | Cost-effective and easy to fabricate | Less durable and requires maintenance | Low |
This strategic material selection guide provides a comprehensive overview of materials suitable for stability chambers, helping international B2B buyers make informed decisions based on their specific needs and regional considerations.
In-depth Look: Manufacturing Processes and Quality Assurance for stability chambers
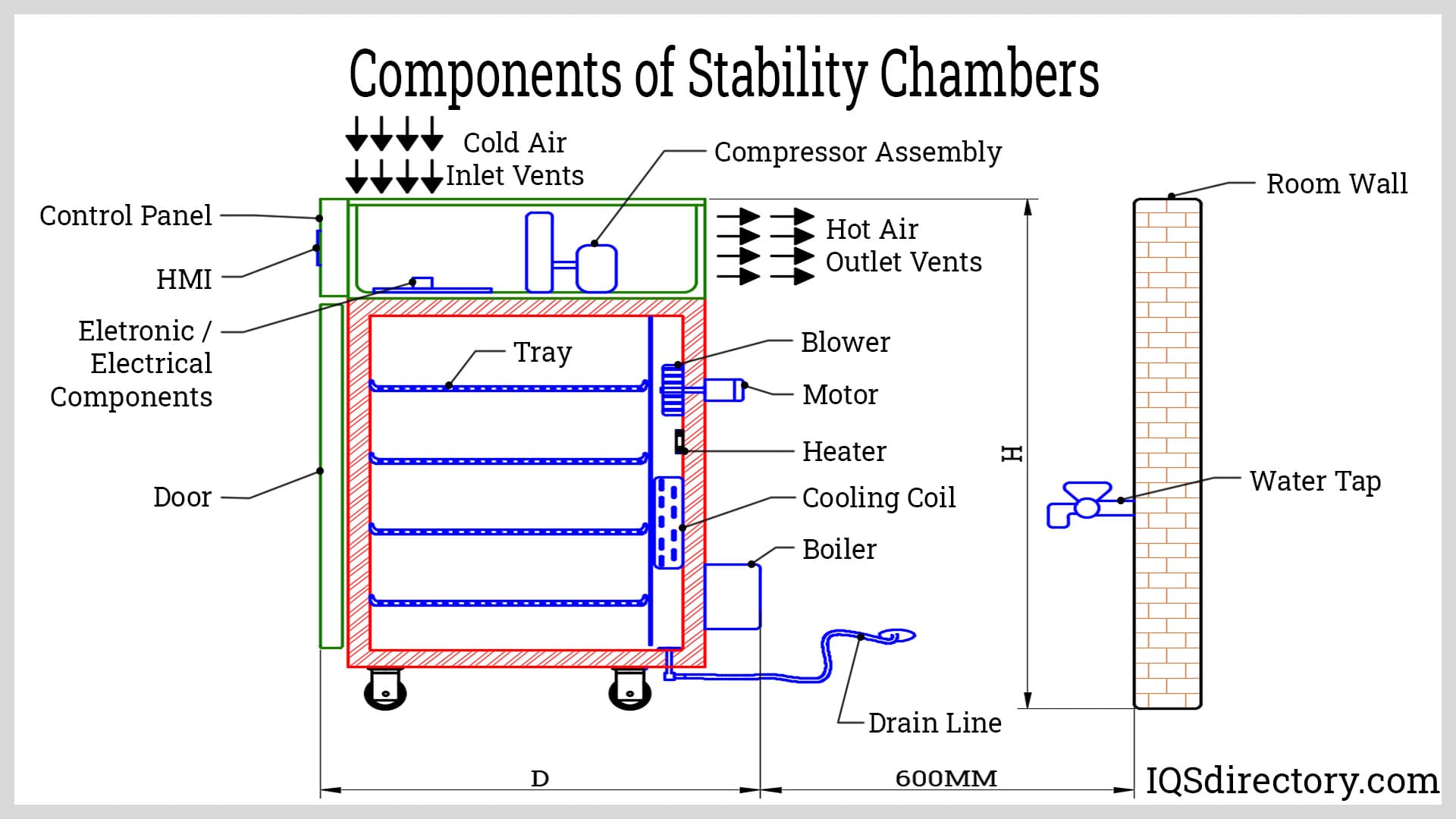
What Are the Main Stages in the Manufacturing Process of Stability Chambers?
The manufacturing process of stability chambers involves several critical stages that ensure the final product meets the stringent requirements for precision and reliability.
-
Material Preparation:
The first step is sourcing high-quality materials, including stainless steel for the chamber’s structure, insulation materials, and electronic components. Manufacturers often utilize advanced materials that provide durability and thermal efficiency. The preparation phase includes quality checks on raw materials to ensure they meet predefined specifications. -
Forming:
In this stage, the prepared materials are shaped into the required components. Techniques such as laser cutting, bending, and welding are commonly employed to create the chamber’s body and internal structures. Precision is paramount in this stage, as any deviations can affect the chamber’s ability to maintain the specified environmental conditions. -
Assembly:
After forming, the components are assembled into a complete unit. This involves integrating the temperature and humidity control systems, sensors, and safety features. During assembly, manufacturers adhere to specific guidelines to ensure correct installation and functionality. This stage often includes detailed inspections to verify that all parts fit correctly and that connections are secure. -
Finishing:
The final stage involves aesthetic and functional finishing processes. This includes painting or powder coating the outer surfaces for corrosion resistance and visual appeal. Additionally, manufacturers conduct thorough cleaning and sterilization to prepare the chambers for use in sensitive environments.
How Do Manufacturers Ensure Quality Control in Stability Chambers?
Quality control (QC) is integral to the manufacturing process of stability chambers, ensuring that each unit performs reliably under specified conditions. Manufacturers typically adhere to various international standards and industry-specific regulations.

Illustrative image related to stability chambers
-
International Standards:
Compliance with ISO 9001 is crucial, as it provides a framework for quality management systems. This certification emphasizes continuous improvement, customer satisfaction, and process optimization. In addition, stability chambers must often meet CE marking requirements to ensure safety and performance in the European market. -
Industry-Specific Regulations:
For pharmaceutical applications, adherence to guidelines set forth by the International Conference on Harmonization (ICH) is vital. These guidelines dictate the standards for stability testing of drug substances and products, ensuring that chambers are capable of maintaining the necessary environmental conditions. -
QC Checkpoints:
Quality control is conducted at various checkpoints throughout the manufacturing process:
– Incoming Quality Control (IQC): Raw materials are inspected upon arrival to ensure they meet specifications.
– In-Process Quality Control (IPQC): Ongoing inspections during the manufacturing process help identify any deviations early, allowing for prompt corrective actions.
– Final Quality Control (FQC): Once assembly is complete, the chamber undergoes comprehensive testing to verify performance against established parameters. -
Common Testing Methods:
Manufacturers utilize several testing methods, including thermal mapping, humidity profiling, and performance testing under simulated conditions. These tests ensure that the chambers can consistently maintain the required temperature and humidity levels across the specified range.
How Can B2B Buyers Verify Supplier Quality Control?
For B2B buyers, particularly those from diverse regions like Africa, South America, the Middle East, and Europe, verifying the quality control measures of potential suppliers is crucial.
-
Supplier Audits:
Conducting audits of suppliers is an effective way to assess their manufacturing processes and quality control systems. Buyers can request to observe the production line, review documentation, and assess compliance with industry standards. -
Quality Control Reports:
Requesting detailed QC reports from suppliers can provide insights into their testing protocols and results. These reports should include information on materials used, testing methods applied, and any certifications obtained. -
Third-Party Inspections:
Engaging third-party inspection services can add an additional layer of assurance. These independent organizations can conduct thorough evaluations of suppliers’ facilities, processes, and products, ensuring compliance with international standards.
What Are the Specific Quality Control and Certification Nuances for International Buyers?
When sourcing stability chambers from international suppliers, buyers should be aware of specific nuances in quality control and certification that can vary by region.
-
Regional Standards Compliance:
Different regions may have unique regulatory requirements that suppliers must meet. For instance, buyers from the European Union should ensure that suppliers have CE certifications, while those in the Middle East may need to comply with local health and safety standards. -
Documentation and Traceability:
International buyers should prioritize suppliers that provide comprehensive documentation regarding manufacturing processes, quality checks, and compliance certifications. This documentation is essential for regulatory submissions and product traceability. -
Cultural and Communication Considerations:
Cultural differences may affect the way quality control processes are perceived and executed. Buyers should establish clear communication channels and expectations regarding quality standards, timelines, and reporting. -
After-Sales Support and Warranty:
Understanding the warranty and support policies of suppliers is vital, especially for international transactions. Buyers should inquire about the terms of warranty, availability of spare parts, and customer support services to ensure they can address any issues that arise post-purchase.
Conclusion
Navigating the manufacturing processes and quality assurance of stability chambers requires a keen understanding of the intricacies involved. By focusing on detailed manufacturing stages, rigorous quality control measures, and verification methods, B2B buyers can make informed decisions that align with their specific testing needs and regulatory requirements. Establishing strong partnerships with reliable suppliers can ultimately lead to successful outcomes in stability testing and product development.
Practical Sourcing Guide: A Step-by-Step Checklist for ‘stability chambers’
Introduction
This practical sourcing guide aims to assist B2B buyers in procuring stability chambers, essential equipment for ensuring the integrity of pharmaceutical products and other sensitive materials. By following this step-by-step checklist, you can make informed decisions that align with your operational needs and regulatory requirements.
Step 1: Define Your Technical Specifications
Before initiating the procurement process, clearly outline the technical specifications required for your stability chamber. Consider factors such as temperature and humidity control ranges, size, and specific applications, like drug stability testing or shelf-life studies. This clarity will help in narrowing down options that best fit your laboratory requirements.
Step 2: Understand Regulatory Compliance Requirements
Ensure that the stability chambers you are considering meet relevant regulatory standards, such as ICH guidelines. Compliance with these regulations is crucial for maintaining product quality and safety, especially in pharmaceutical applications. Verify certifications and documentation that confirm adherence to these standards.
Step 3: Evaluate Potential Suppliers
Conduct thorough research on potential suppliers. Request detailed company profiles, product catalogs, and case studies that highlight their experience in providing stability chambers. Look for suppliers with positive reviews and references from similar industries or regions to gauge their reliability and support capabilities.
Step 4: Assess Customization Options
Stability chambers often require customization to meet specific testing protocols. Inquire about the availability of features such as adjustable shelving, access ports, and humidity systems tailored to your laboratory’s needs. Customization can enhance the efficiency and effectiveness of your testing procedures.

Illustrative image related to stability chambers
Step 5: Compare Pricing and Total Cost of Ownership
While initial pricing is important, consider the total cost of ownership, which includes maintenance, consumables, and energy efficiency. Request quotes that outline all costs associated with the chambers, including installation and training services. This comprehensive view will help you make a more economically sound decision.
Step 6: Verify After-Sales Support and Warranty Terms
Evaluate the after-sales support offered by suppliers, including warranty terms and maintenance services. A robust support system can significantly reduce downtime and extend the lifespan of your stability chamber. Look for suppliers that provide training, technical assistance, and readily available spare parts.
Step 7: Seek Demonstrations and Trials
Whenever possible, request demonstrations or trials of the stability chambers you are considering. This hands-on experience allows you to evaluate the equipment’s functionality, ease of use, and compatibility with your existing systems. Observing the chamber in action can provide insights that specifications alone may not reveal.
By following these steps, you will be better equipped to procure stability chambers that meet your operational needs and uphold the quality standards essential in your industry.
Comprehensive Cost and Pricing Analysis for stability chambers Sourcing
What Are the Key Cost Components in Stability Chambers?
When evaluating the cost structure of stability chambers, several components play critical roles:
-
Materials: The choice of materials significantly impacts costs. High-quality stainless steel, specialized insulation, and advanced electronic components contribute to durability and performance, but also increase expenses. The cost of these materials can fluctuate based on market conditions, affecting overall pricing.
-
Labor: Skilled labor is necessary for manufacturing stability chambers, especially for custom designs. Labor costs vary by region; for example, manufacturing in Europe might incur higher labor costs compared to facilities in Asia or Africa.
-
Manufacturing Overhead: This includes costs related to factory operations, utilities, maintenance, and equipment depreciation. Efficient manufacturing processes can help reduce overhead costs, but these savings might not always translate to lower prices for buyers.
-
Tooling: Custom tooling for specific chamber designs or features can add substantial costs. Buyers should consider whether they require standard models or if custom tooling is necessary, as this can significantly influence the total cost.
-
Quality Control (QC): Ensuring compliance with international standards such as ICH guidelines requires rigorous testing and quality assurance processes, which can increase costs. Manufacturers often pass these expenses onto buyers.
-
Logistics: Shipping stability chambers internationally involves freight costs, customs duties, and potential tariffs. The complexity of logistics can vary greatly depending on the destination, influencing the total cost.
-
Margin: Supplier margins can vary widely based on market positioning, brand reputation, and product differentiation. Established brands with a reputation for reliability may command higher prices.
How Do Price Influencers Affect Stability Chamber Costs?
Several factors can influence the pricing of stability chambers:
-
Volume/MOQ (Minimum Order Quantity): Larger orders typically lead to reduced per-unit costs due to economies of scale. Buyers may negotiate better pricing with suppliers when purchasing in bulk.
-
Specifications and Customization: Custom features such as specific temperature and humidity ranges, chamber sizes, and additional functionalities can significantly raise costs. Buyers should clearly define their requirements to avoid unexpected expenses.
-
Material Quality and Certifications: Chambers that meet higher quality standards or possess certifications (e.g., ISO, CE) often come at a premium. Buyers must assess whether these certifications are necessary for their applications.
-
Supplier Factors: Reputation, reliability, and after-sales support are crucial. Established suppliers may offer higher prices but can provide superior service and warranty terms.
-
Incoterms: Understanding shipping terms is vital for international transactions. The chosen Incoterms can affect the buyer’s total cost, as they dictate who bears the risk and costs during transport.
What Tips Can Help Buyers Optimize Costs?
B2B buyers should consider the following strategies to maximize cost-efficiency when sourcing stability chambers:
-
Negotiation Strategies: Engage suppliers in discussions to find mutually beneficial terms. Leveraging competitive quotes can enhance negotiation power.
-
Understanding Total Cost of Ownership (TCO): TCO encompasses not just the purchase price but also maintenance, operational costs, and potential downtimes. Buyers should evaluate the long-term costs associated with the chambers.
-
International Pricing Nuances: Buyers from regions like Africa, South America, and the Middle East should be aware of local tariffs, taxes, and import duties that can impact final costs. Engaging with suppliers who understand these nuances can prevent unforeseen expenses.
-
Consider Local Suppliers: Sourcing from local manufacturers can reduce shipping costs and lead times, providing a more cost-effective solution compared to international sourcing.
Disclaimer on Pricing
Prices for stability chambers can vary widely based on specifications, supplier, and market conditions. The figures provided in this analysis are indicative and should be verified with suppliers for accurate quotations tailored to specific needs. Always consider obtaining multiple quotes to ensure competitive pricing.

Illustrative image related to stability chambers
Alternatives Analysis: Comparing stability chambers With Other Solutions
When considering solutions for environmental testing and stability studies, it is essential to explore alternatives to stability chambers. These alternatives may offer varying benefits based on specific requirements, such as budget constraints, space limitations, or particular testing needs. Below, we compare stability chambers with two viable alternatives: climate-controlled rooms and desiccators.
| Comparison Aspect | Stability Chambers | Climate-Controlled Rooms | Desiccators |
|---|---|---|---|
| Performance | High precision in temperature and humidity control, compliant with ICH guidelines | Excellent for large-scale testing; customizable for various environmental conditions | Effective for low humidity environments; suitable for small samples |
| Cost | Higher initial investment and operational costs due to advanced technology | Often more economical for larger operations but can vary widely based on customization | Low-cost option for moisture-sensitive materials, but limited in scope |
| Ease of Implementation | Requires specialized installation and setup; user training necessary | May require significant construction; adaptable for multiple uses | Simple setup with minimal installation; portable options available |
| Maintenance | Regular calibration and water quality management needed | Maintenance can be intensive depending on size and complexity | Low maintenance; periodic checks needed for seals and desiccants |
| Best Use Case | Ideal for pharmaceuticals, food products, and sensitive biological materials | Suited for large laboratories needing comprehensive environmental control | Best for small-scale applications where moisture control is critical |
What are the Pros and Cons of Climate-Controlled Rooms as an Alternative?
Climate-controlled rooms provide a significant alternative to stability chambers, particularly for organizations that require extensive testing capabilities. These rooms can be customized to meet specific temperature and humidity conditions, making them suitable for a range of applications. The primary advantage is their ability to accommodate larger volumes of products or materials, which is beneficial for companies with high throughput needs. However, the initial setup costs can be substantial, especially if extensive renovations are required. Additionally, maintaining precise environmental controls may demand more resources compared to dedicated stability chambers.
How Do Desiccators Function as a Cost-Effective Option?
Desiccators serve as a low-cost solution for controlling humidity levels, particularly for smaller samples or materials that are sensitive to moisture. They work by using desiccants to absorb moisture, creating a dry environment. The advantages include minimal operational costs and straightforward maintenance, making them ideal for laboratories with limited budgets. However, desiccators are not suitable for large-scale testing or for applications that require precise temperature control. Their scope is limited compared to stability chambers and climate-controlled rooms, which can handle a broader range of testing requirements.
How Can B2B Buyers Choose the Right Solution for Their Needs?
In selecting the appropriate solution for environmental testing, B2B buyers should evaluate their specific needs, including the scale of testing, budget constraints, and regulatory requirements. Stability chambers are often the best choice for industries that demand high precision and compliance with guidelines such as ICH. In contrast, climate-controlled rooms may be more suitable for larger operations that require flexibility and capacity. Desiccators are excellent for niche applications where cost-effectiveness is crucial. Ultimately, understanding the unique requirements of their operations will guide buyers in making an informed decision that aligns with their testing objectives and operational goals.
Essential Technical Properties and Trade Terminology for stability chambers
What Are the Essential Technical Properties of Stability Chambers?
Stability chambers are critical for ensuring the quality and efficacy of pharmaceutical products, biologics, and various packaged goods. Understanding the essential technical properties can help B2B buyers make informed purchasing decisions.
1. Temperature Control Range: Why Is It Important?
The temperature control range of stability chambers typically spans from 0°C to 60°C (32°F to 140°F). This specification is vital for simulating various storage conditions that products may encounter throughout their lifecycle. Accurate temperature control ensures that products are tested under realistic scenarios, thus guaranteeing their stability and safety.
2. Humidity Control Range: What Should You Look For?
Humidity control is another crucial property, often maintained from above ambient levels to 95% relative humidity at specific temperatures, such as 37°C. This feature is essential for stability testing, as many products are sensitive to moisture. A precise humidity control system reduces the risk of product degradation, ensuring compliance with industry standards and regulations.
3. Size and Capacity: How Do They Affect Your Operations?
Stability chambers come in various sizes, typically measured in cubic feet (e.g., 10 cu. ft., 50 cu. ft.). The choice of size depends on your laboratory’s specific needs, such as the volume of products to be tested. Larger chambers can accommodate more samples, enhancing efficiency, while smaller models are suitable for limited space or fewer samples.
4. Material and Build Quality: Why Do They Matter?
The materials used in constructing stability chambers often include high-grade stainless steel and insulated panels. These materials not only ensure durability but also help maintain internal conditions by minimizing external temperature fluctuations. Investing in high-quality construction can lead to long-term savings by reducing maintenance costs and prolonging the lifespan of the equipment.
5. Regulatory Compliance: What Standards Should You Consider?
Stability chambers should meet specific regulatory guidelines, such as those set by the International Conference on Harmonization (ICH). Compliance ensures that the equipment can be used for drug stability studies and other critical applications. Buyers must verify that the chambers adhere to these standards to avoid legal repercussions and ensure the validity of their testing results.
What Are Common Trade Terms Used in Stability Chambers?
Familiarity with industry jargon can streamline communication and negotiations between buyers and suppliers. Here are some key terms that are commonly encountered in the stability chamber market.
1. OEM (Original Equipment Manufacturer): What Does It Mean?
An OEM refers to a company that produces parts or equipment that may be marketed by another manufacturer. In the context of stability chambers, understanding whether the supplier is an OEM can help buyers assess the quality and reliability of the equipment.
2. MOQ (Minimum Order Quantity): How Does It Impact Your Purchase?
MOQ is the smallest quantity of a product that a supplier is willing to sell. Knowing the MOQ is crucial for budgeting and inventory management, especially for smaller companies that may not need large quantities of chambers.
3. RFQ (Request for Quotation): What Is Its Purpose?
An RFQ is a document sent to suppliers requesting pricing and other details for specific products. Submitting an RFQ can help buyers compare offers and negotiate better deals, ensuring they get the best value for their investment.
4. Incoterms: What Are They and Why Are They Important?
Incoterms are international commercial terms that define the responsibilities of buyers and sellers in shipping goods. Familiarity with these terms can help buyers understand shipping costs, risks, and liabilities, leading to smoother transactions.
5. Calibration Certificate: Why Is It Necessary?
A calibration certificate verifies that a stability chamber has been tested and meets specified performance criteria. This document is essential for ensuring compliance with regulatory standards and should be requested before purchase.
By understanding these technical properties and trade terms, B2B buyers can navigate the complexities of purchasing stability chambers more effectively, ensuring they select the right equipment for their operational needs.
Navigating Market Dynamics and Sourcing Trends in the stability chambers Sector
Global drivers impacting the stability chambers market include increased regulatory scrutiny and the growing need for reliable storage solutions in pharmaceuticals, food, and environmental testing. Compliance with International Conference on Harmonization (ICH) guidelines is paramount for international buyers, particularly in regions like Africa, South America, the Middle East, and Europe, where pharmaceutical markets are expanding. This compliance drives the demand for high-quality stability chambers capable of precise temperature and humidity control, which are essential for conducting drug stability studies and shelf-life testing.
Emerging B2B tech trends are significantly shaping the sourcing landscape. Automation and IoT integration are gaining traction, allowing for real-time monitoring and data analytics. This tech not only enhances operational efficiency but also ensures compliance with stringent regulations by providing accurate and timely data. Additionally, the trend toward modular and customizable solutions is becoming popular, allowing labs to tailor their chambers to specific testing requirements, thus optimizing space and resources.
In terms of market dynamics, buyers must navigate a competitive landscape with various manufacturers offering a wide range of products. Price sensitivity remains a significant factor, particularly for buyers in developing regions. However, investing in high-quality chambers can lead to long-term cost savings through improved reliability and reduced maintenance. International buyers should also consider factors such as supplier reputation, after-sales service, and warranty options when sourcing stability chambers to ensure optimal performance and compliance.

Illustrative image related to stability chambers
How Can Sustainability Influence B2B Sourcing of Stability Chambers?
Sustainability and ethical sourcing are increasingly important in the stability chambers sector. Environmental impact concerns are driving manufacturers to adopt greener practices in production and materials. Buyers are now looking for suppliers who prioritize sustainability, including the use of recyclable materials and energy-efficient designs that minimize carbon footprints.
Incorporating ‘green’ certifications into the sourcing process can add value for B2B buyers. Certifications such as ISO 14001 for environmental management systems indicate a commitment to sustainable practices. Buyers should also consider the lifecycle impacts of the equipment, from production to end-of-life disposal. By prioritizing suppliers that demonstrate sustainable practices, companies can enhance their brand reputation and comply with emerging regulations aimed at reducing environmental harm.
Furthermore, ethical supply chains are essential in ensuring fair labor practices and responsible sourcing of materials. Buyers should engage with suppliers who transparently communicate their sourcing strategies and provide documentation of ethical practices. This not only builds trust but also aligns with the growing consumer demand for responsible and ethical business practices.

Illustrative image related to stability chambers
What is the Historical Context of Stability Chambers in B2B Markets?
The evolution of stability chambers can be traced back to the increasing need for controlled environments in laboratories and manufacturing facilities. Initially designed for basic temperature control, advancements in technology have transformed these chambers into sophisticated systems capable of precise temperature, humidity, and light control.
The introduction of regulatory standards, particularly in the pharmaceutical industry, spurred the development of advanced stability chambers to meet compliance requirements. Over the years, manufacturers have incorporated features such as automation, real-time monitoring, and customizable options, allowing for tailored solutions that meet specific testing needs. This historical context highlights the importance of innovation in maintaining compliance and ensuring product integrity, making stability chambers an integral component of modern laboratory operations.
In summary, international B2B buyers in the stability chambers sector must navigate evolving market dynamics, prioritize sustainability, and understand the historical context to make informed sourcing decisions. This comprehensive approach not only ensures compliance and reliability but also positions companies as leaders in responsible business practices.

Illustrative image related to stability chambers
Frequently Asked Questions (FAQs) for B2B Buyers of stability chambers
-
How do I ensure my stability chamber meets regulatory compliance?
To ensure your stability chamber meets regulatory compliance, particularly the International Conference on Harmonization (ICH) guidelines, start by selecting a chamber specifically designed for ICH stability testing. Verify that the manufacturer provides appropriate documentation, including calibration certificates and performance specifications. Regular maintenance, temperature mapping, and humidity control checks are crucial to maintain compliance. Additionally, engage with suppliers who can offer ongoing support and training to ensure your team understands the operational requirements and documentation processes. -
What features should I look for in a stability chamber for pharmaceutical testing?
When sourcing a stability chamber for pharmaceutical testing, prioritize features such as precise temperature control within the range of 0°C to 60°C and humidity control up to 95% RH. Look for chambers with advanced data logging capabilities to track and record conditions over time. Ensure the unit has customizable options like shelving, access ports, and various door configurations to accommodate your specific testing needs. Furthermore, consider energy efficiency and ease of maintenance, as these factors can significantly impact operational costs. -
How can I customize a stability chamber to fit my specific needs?
Most manufacturers offer customization options for stability chambers, allowing you to tailor the unit to your specific testing requirements. Customizations may include size adjustments, specific temperature and humidity settings, and additional features such as shelving or access ports. Discuss your unique needs with potential suppliers to explore available options. Ensure that any modifications still align with regulatory standards and that the manufacturer can provide necessary validation for the customized features. -
What are the minimum order quantities (MOQs) for stability chambers?
Minimum order quantities for stability chambers can vary significantly by manufacturer and region. Some suppliers may have MOQs as low as one unit, especially for standard models, while others may require larger orders for customized or specialized units. It’s essential to clarify the MOQ with potential suppliers during the negotiation phase. If you’re a smaller buyer, consider forming a consortium with other businesses to meet MOQ requirements while benefiting from bulk pricing. -
What payment terms should I expect when purchasing stability chambers internationally?
Payment terms for international purchases of stability chambers can vary widely. Common options include advance payment, letters of credit, or payment upon delivery. Many suppliers may also offer installment plans, especially for higher-value equipment. It’s crucial to discuss and negotiate payment terms upfront to ensure they align with your financial processes. Be aware of additional costs such as shipping, customs duties, and taxes, which should be factored into your budget. -
How do I vet suppliers for stability chambers in international markets?
To effectively vet suppliers for stability chambers, conduct thorough research to assess their reputation and reliability. Check for certifications such as ISO 9001, which indicates quality management standards. Request references from other B2B clients, particularly those in your industry. Additionally, consider visiting the supplier’s manufacturing facility if possible, or use third-party audits to gain insights into their operational capabilities. Online platforms and trade shows can also provide valuable networking opportunities to meet and evaluate potential suppliers. -
What logistics considerations should I be aware of when importing stability chambers?
When importing stability chambers, logistics considerations include shipping methods, customs clearance, and potential tariffs. Choose a reliable freight forwarder with experience in handling laboratory equipment to ensure safe transport. Be prepared for potential delays at customs, and ensure all documentation is complete, including invoices, packing lists, and compliance certificates. Also, consider the environmental conditions during transit, as stability chambers require specific handling to avoid damage. Proper insurance coverage during shipping is also advisable to mitigate risks. -
What should I know about the warranty and after-sales support for stability chambers?
Understanding the warranty and after-sales support for stability chambers is crucial for long-term operational success. Most manufacturers offer warranties ranging from one to five years, covering parts and labor for defects. Inquire about the details of the warranty, including what is covered and any exclusions. Additionally, assess the availability of after-sales support, such as technical assistance, maintenance services, and training for your team. Reliable after-sales support can significantly reduce downtime and ensure your equipment operates optimally.
Top 5 Stability Chambers Manufacturers & Suppliers List
1. BSILAB – Stability Testing Chambers
Domain: bsilab.com
Registered: 2009 (16 years)
Introduction: This company, BSILAB – Stability Testing Chambers, is a notable entity in the market. For specific product details, it is recommended to visit their website directly.
2. CSZ Industrial – Stability Chambers
Domain: cszindustrial.com
Registered: 2002 (23 years)
Introduction: Stability Chambers are used to test and store a wide range of products in specific temperature and/or humidity conditions. Applications include shelf life testing, stability testing, expiration date testing, and accelerated aging. Ideal for industries such as pharmaceutical, packaging, life science, personal care, medical, and biomedical storage. The PharmaEvent stability chambers feature benchtop…
3. Caron Scientific – Environmental Stability Chambers
Domain: caronscientific.com
Registered: 2024 (1 years)
Introduction: Stability/Environmental Chambers: 10-75 cu.ft (283-2124L), Temp range 5 – 70C, Controlled RH to 95%. Heated/Humidified Chambers: 10 – 33 cu.ft (283 – 934L), Temp range 10C above ambient to 70C, Controlled RH to 95%. Freeze/Thaw Chambers: 25 – 33 cu.ft (708 – 934L), Temp range -25 to 70C, Controlled RH to 95%. Photostability Chamber: 11 cu.ft (311L), Temp range 10 – 35C, Controlled Visible; UV ligh…
4. BMT USA – ICH Stability Chambers
Domain: bmtusa.com
Registered: 2009 (16 years)
Introduction: ICH Stability Chambers provide precise control of temperature, humidity, and light for accurate ICH Q1A stability and Q1B photostability testing of pharmaceutical and cosmetic products. Suitable for shelf life studies of food and beverage products and freeze-thaw testing. Features include a patented forced air convection system for rapid heating and cooling with uniform temperature, UV/VIS shelf a…
5. Thomas Scientific – Humidity and Temperature Stability Chambers
Domain: thomassci.com
Registered: 1995 (30 years)
Introduction: Humidity and Temperature Stability Chambers
Controller Information:
– Proportional integral derivative (PID) controller with LCD display
– Programmable ramp and soak
– Process phase graphic animations
– USB data port
– RS-485 communication (MODBUS-RTU)
– Real time clock
– Battery backup
– Event logs: 100
– Visual alarm indicator
– Audible alarm indicator
– Process phase specific temperature alarm…
Strategic Sourcing Conclusion and Outlook for stability chambers
How Can Strategic Sourcing of Stability Chambers Enhance Your Business?
In the competitive landscape of pharmaceuticals and biotechnology, the strategic sourcing of stability chambers is crucial for ensuring compliance and maintaining product integrity. By investing in high-quality stability chambers that meet International Conference on Harmonization (ICH) guidelines, businesses can conduct reliable drug stability studies and shelf-life testing. This not only enhances operational efficiency but also reduces the risk of costly regulatory setbacks.
International B2B buyers, particularly from regions like Africa, South America, the Middle East, and Europe, must prioritize suppliers that offer customizable options and robust after-sales support. The ability to tailor chamber specifications to unique testing requirements—such as temperature and humidity controls—can significantly impact research outcomes and product quality.
Looking ahead, the demand for advanced stability testing solutions will only grow. Companies that adopt a proactive approach to sourcing these critical assets will position themselves for long-term success. Engage with reputable suppliers, assess your specific needs, and ensure your laboratory is equipped with the best technology to meet emerging challenges. Embrace this opportunity to enhance your capabilities and stay ahead in a rapidly evolving market.
Important Disclaimer & Terms of Use
⚠️ Important Disclaimer
The information provided in this guide, including content regarding manufacturers, technical specifications, and market analysis, is for informational and educational purposes only. It does not constitute professional procurement advice, financial advice, or legal advice.
While we have made every effort to ensure the accuracy and timeliness of the information, we are not responsible for any errors, omissions, or outdated information. Market conditions, company details, and technical standards are subject to change.
B2B buyers must conduct their own independent and thorough due diligence before making any purchasing decisions. This includes contacting suppliers directly, verifying certifications, requesting samples, and seeking professional consultation. The risk of relying on any information in this guide is borne solely by the reader.